Abstract
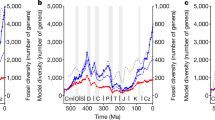
The unprecedented diversifications in the fossil record of the early Palaeozoic (541–419 million years ago) increased both within-sample (α) and global (γ) diversity, generating considerable ecological complexity. Faunal difference (β diversity), including spatial heterogeneity, is thought to have played a major role in early Palaeozoic marine diversification, although α diversity is the major determinant of γ diversity through the Phanerozoic. Drivers for this Phanerozoic shift from β to α diversity are not yet resolved. Here, we evaluate the impacts of environmental and faunal heterogeneity on diversity patterns using a global spatial grid. We present early Palaeozoic genus-level α, β and γ diversity curves for molluscs, brachiopods, trilobites and echinoderms and compare them with measures of spatial lithological heterogeneity, which is our proxy for environmental heterogeneity. We find that α and β diversity are associated with increased lithological heterogeneity, and that β diversity declines over time while α increases. We suggest that the enhanced dispersal of marine taxa from the Middle Ordovician onwards facilitated increases in α diversity by encouraging the occupation of narrow niches and increasing the prevalence of transient species, simultaneously reducing spatial β diversity. This may have contributed to a shift from β to α diversity as the major determinant of γ diversity increase over this critical evolutionary interval.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used in this work can be downloaded from the PBDB (https://paleobiodb.org/#/). The direct links for retrieving the data used to generate these results are available in the accompanying code (see the code availability).
Code availability
The complete code and relevant results are recorded in R code and can be downloaded via Zeonodo (https://doi.org/10.5281/zenodo.3463219).
References
Sepkoski, J. J. Alpha, beta, or gamma: where does all the diversity go? Paleobiology 14, 221–234 (1988).
Servais, T., Owen, A. W., Harper, D. A. T., Kröger, B. & Munnecke, A. The Great Ordovician Biodiversification Event (GOBE): the palaeoecological dimension. Palaeogeogr. Palaeoclimatol. Palaeoecol. 294, 99–119 (2010).
Servais, T. et al. The onset of the ‘Ordovician Plankton Revolution’ in the Late Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 458, 12–28 (2016).
Smith, M. P. & Harper, D. A. T. Causes of the Cambrian explosion. Science 341, 1355–1356 (2013).
Harper, D. A. T. The Ordovician biodiversification: setting an agenda for marine life. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 148–166 (2006).
Droser, M. L. & Finnegan, S. The Ordovician Radiation: a follow-up to the Cambrian Explosion? Integr. Comp. Biol. 43, 178–184 (2003).
Rasmussen, C. M. Ø., Kröger, B., Nielsen, M. L. & Colmenar, J. Cascading trend of Early Paleozoic marine radiations paused by Late Ordovician extinctions. Proc. Natl Acad. Sci. USA 116, 7207–7213 (2019).
Servais, T. & Harper, D. A. T. The Great Ordovician Biodiversification Event (GOBE): definition, concept and duration. Lethaia 51, 151–164 (2018).
Webby, B. D., Paris, F., Droser, M. L. & Percival, I. G. The Great Ordovician Biodiversification Event (Columbia Univ. Press, 2004).
Stigall, A. L., Edwards, C. T., Freeman, R. L. & Rasmussen, C. M. Ø. Coordinated biotic and abiotic change during the Great Ordovician Biodiversification Event: darriwilian assembly of early paleozoic building blocks. Palaeogeogr. Palaeoclimatol. Palaeoecol. 530, 249–270 (2019).
Erwin, D. H. & Valentine, J. W. The Cambrian Explosion: The Construction of Animal Biodiversity (Roberts and Company Publishers, 2013).
Stigall, A. L. Ordovician oxygen and biodiversity. Nat. Geosci. 10, 883–888 (2017).
Na, L. & Kiessling, W. Diversity partitioning during the Cambrian radiation. Proc. Natl Acad. Sci. USA 112, 4702–4706 (2015).
Hofmann, R., Tietje, M. & Aberhan, M. Diversity partitioning in Phanerozoic benthic marine communities. Proc. Natl Acad. Sci. USA 116, 79–83 (2019).
Miller, A. I. Dissecting global diversity patterns: examples from the Ordovician Radiation. Annu. Rev. Ecol. Syst. 28, 85–104 (1997).
Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 88, 2427–2439 (2007).
Harper, D. A. T. The Ordovician brachiopod radiation: roles of alpha, beta, and gamma diversity. Geol. Soc. Am. Spec. Pap. 466, 69–83 (2010).
Alroy, J. et al. Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100 (2008).
Hautmann, M. Diversification and diversity partitioning. Paleobiology 40, 162–176 (2014).
Miller, A. I. et al. Phanerozoic trends in the global geographic disparity of marine biotas. Paleobiology 35, 612–630 (2009).
Stigall, A. L. How is biodiversity produced? Examining speciation processes during the GOBE. Lethaia 51, 165–172 (2018).
Stigall, A. L., Bauer, J. E., Lam, A. R. & Wright, D. F. Biotic immigration events, speciation, and the accumulation of biodiversity in the fossil record. Glob. Planet. Change 148, 242–257 (2017).
Miller, A. I. A new look at age and area: the geographic and environmental expansion of genera during the Ordovician radiation. Paleobiology 23, 410–419 (1997).
Miller, A. I. & Mao, S. in Biodiversity Dynamics: Turnover of Populations, Taxa, and Communities (eds McKinney, M. L. & Drake, J. A.) Ch. 13 (Columbia Univ. Press, 2001).
Zaffos, A., Finnegan, S. & Peters, S. E. Plate tectonic regulation of global marine animal diversity. Proc. Natl. Acad. Sci. USA 114, 5653–5658 (2017).
Kröger, B. Changes in the latitudinal diversity gradient during the Great Ordovician Biodiversification Event. Geology 46, 44–47 (2017).
Darroch, S. A. F. & Wagner, P. J. Response of beta diversity to pulses of Ordovician-Silurian mass extinction. Ecology 96, 532–549 (2015).
Kröger, B. & Lintulaakso, K. RNames, a stratigraphical database designed for the statistical analysis of fossil occurrences–the Ordovician diversification as a case study. Palaeontol. Electron. 20, 20.1.1T (2017).
Jaanusson, V. & Bergström, S. M. Middle Ordovician faunal spatial differentiation in Baltoscandia and the Appalachians. Alcheringa 4, 89–110 (1980).
Kröger, B. in Early Palaeozoic Biogeography and Palaeogeography Geological Society Memoir No. 38 (eds Harper, D. A. T. & Servais, T.) 429–448 (Geological Society of London, 2013).
Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010).
Harper, D. A. T. & Servais, T. in Early Palaeozoic Biogeography and Palaeogeography Geological Society Memoir No. 38 (eds Harper, D. A. T. & Servais, T.) 1–4 (Geological Society of London, 2013).
Harper, D. A. T. et al. in Early Palaeozoic Biogeography and Palaeogeography Geological Society Memoir No. 38 (eds Harper, D. A. T. & Servais, T.) 127–144 (Geological Society of London, 2013).
Cocks, L. R. M. & Fortey, R. A. in Palaeozoic Palaeogeography and Biogeography Geological Society Memoir No. 12 (eds McKerrow, W. S. & Scotese, C. R.) 97–104 (Geological Society of London, 1990).
Nützel, A., Lehnert, O. & Frýda, J. Origin of planktotrophy- evidence from early molluscs. Evol. Dev. 8, 325–330 (2006).
Peterson, K. J. Macroevolutionary interplay between planktic larvae and benthic predators. Geology 33, 929–932 (2005).
Jablonski, D. & Lutz, R. A. Larval ecology of marine benthic invertebrates: paleobiological implications. Biol. Rev. 58, 21–89 (1983).
Lam, A. R., Stigall, A. L. & Matzke, N. J. Dispersal in the Ordovician: speciation patterns and paleobiogeographic analyses of brachiopods and trilobites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 489, 147–165 (2017).
Kröger, B. & Aubrechtová, M. The cephalopods of the Kullsberg Limestone Formation, Upper Ordovician, central Sweden and the effects of reef diversification on cephalopod diversity. J. Syst. Palaeontol. 17, 961–995 (2019).
McPeek, M. A. The macroevolutionary consequences of ecological differences among species. Palaeontology 50, 111–129 (2007).
McPeek, M. A. The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270–E284 (2008).
Leibold, M. A. & McPeek, M. A. Coexistence of the niche and neutral perspectives in community ecology. Ecology 87, 1399–1410 (2006).
Kröger, B., Franeck, F. & Rasmussen, C. M. Ø. The evolutionary dynamics of the early Palaeozoic marine biodiversity accumulation. Proc. R. Soc. B 286, 20191634 (2019).
Kocsis, Á. T. Icosa: Global triangular and penta-hexagonal grids based on tessellated icosahedra. R version 0.9.81 https://cran.r-project.org/web/packages/icosa/index.html (2017).
Wright, N. M., Zahirovic, S. & Seton, M. Towards community-driven paleogeographic reconstructions: integrating open-access paleogeographic and paleobiology data with plate tectonics. Biogeosciences 10, 1529–1541 (2013).
Cohen, K. M., Harper, D. A. T. & Gibbard, P. L. ICS International Chronostratigraphic Chart v2018/08 (International Commission on Stratigraphy, IUGS, 2018); http://www.stratigraphy.org
Gradstein, F., Ogg, J., Schmitz, M. & Ogg, G. The Geologic Timescale (Elsevier, 2012).
Alroy, J. Fair sampling of taxonomic richness and unbiased estimation of origination and extinction rates. Paleontol. Soc. Pap. 16, 55–80 (2010).
Tuomisto, H. A diversity of beta diversities: straightening up a concept gone awry. Part 2. Quantifying beta diversity and related phenomena. Ecography 33, 23–45 (2010).
Tuomisto, H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33, 2–22 (2010).
Koleff, P., Gaston, K. J. & Lennon, J. J. Measuring beta diversity for presence-absence data. J. Anim. Ecol. 72, 367–382 (2003).
Barwell, L. J., Isaac, N. J. B. & Kunin, W. E. Measuring β-diversity with species abundance data. J. Anim. Ecol. 84, 1112–1122 (2015).
Anderson, M. J. et al. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28 (2011).
Patzkowsky, M. E. & Holland, S. M. Diversity partitioning of a Late Ordovician marine biotic invasion: controls on diversity in regional ecosystems. Paleobiology 33, 295–309 (2007).
Oksanen, A. J. et al. Vegan: Community ecology package. R version 2.5.2 https://CRAN.R-project.org/package=vegan (2018).
Wright, D. H. A comparative analysis of nested subset patterns of species composition. Oecologia 113, 1–20 (1998).
Lennon, J. J., Koleff, P., Greenwood, J. J. D. & Gaston, K. J. The geographical structure of British bird distributions: diversity, spatial turnover and scale. J. Anim. Ecol. 70, 966–979 (2001).
Simpson, G. G. Mammals and the nature of continents. Am. J. Sci. 241, 1–31 (1943).
Sørensen, T. A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. K. Danske Vidensk. Selsk. Biol. Skr. 5, 1–34 (1948).
Brocklehurst, N., Day, M. O. & Fröbisch, J. Accounting for differences in species frequency distributions when calculating beta diversity in the fossil record. Methods Ecol. Evol. 9, 1409–1420 (2018).
Bray, J. R. & Curtis, J. T. An Ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (1957).
Edwards, C. T., Saltzman, M. R., Royer, D. L. & Fike, D. A. Oxygenation as a driver of the Great Ordovician Biodiversification Event. Nat. Geosci. 10, 925–929 (2017).
Clarke, A. & Gaston, K. J. Climate, energy and diversity. Proc. R. Soc. B 273, 2257–2266 (2006).
Hull, P. M., Darroch, S. A. F. & Erwin, D. H. Rarity in mass extinctions and the future of ecosystems. Nature 528, 345–351 (2015).
Hsieh, T. C., Ma, K. H. & Chao, A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456 (2016).
Hardin, G. The competitive exclusion principle. Science 131, 1292–1297 (1960).
Peters, S. E. & Foote, M. Determinants of extinction in the fossil record. Nature 416, 420–424 (2002).
Peters, S. E. Geologic constraints on the macroevolutionary history of marine animals. Proc. Natl Acad. Sci. USA 102, 12326–12331 (2005).
Smith, A. B. & McGowan, A. J. How much can be predicted from the sedimentary rock record of western Europe? Palaeontology 50, 765–774 (2007).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018); https://www.R-project.org/.
Acknowledgements
This study was part of the Academy of Finland-funded project ‘Ecological engineering as a biodiversity driver in deep time’. We thank S. Scholze for data entry into the PBDB over the course of this study, M. Wale for help with running sensitivity analyses and R. Hofmann for helpful discussions on β diversity in the Palaeozoic. This is a contribution to IGCP 653 (The onset of the Great Ordovician Biodiversification Event).
Author information
Authors and Affiliations
Contributions
A.P. and B.K. devised the research, agreed on analytical techniques and wrote the paper. A.P. calculated β diversity and checked correlations. B.K. downloaded and formatted data from the PBDB, calculated α and γ diversity, and drew the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Raw beta diversity through time.
Raw (non RAC) beta diversity through time, showing high values interpreted to be caused by incomplete sampling.
Extended Data Fig. 2 Sensitivity analysis of the effect of grid size on beta diversity.
RAC beta diversity curves made using a global grid of hexagons with side (a) 55 km and (b) 222 km. Kolmogorov-Smirnov tests show no significant difference between these curves and the curve made with a 111 km grid. (a) D = 0.15, p = 0.99 for grids of side 55km; (b) D = 0.08, p = 1 for grids of side 222 km.
Extended Data Fig. 3 Sensitivity analysis of the effect of standardization coverage on beta diversity.
RAC beta diversity curves made using a standardization coverage of (a) 0.2 and (b) 0.5. Kolmogorov-Smirnov tests show no significant difference between these curves and the curve with a standardization coverage of 0.4 (a) D = 0.23, p = 0.90 for a standardization coverage of 0.2; (b) D = 0.19, p = 0.94 for a standardization coverage of 0.5.
Extended Data Fig. 4 Beta diversity curves made using the Simpson and Bray-Curtis dissimilarities.
RAC beta diversity curves showing (a) the Bray-Curtis and (b) the Simpson dissimilarity through time. Kolmogorov-Smirnov tests show no significant difference between these curves and the RAC Sørensen dissimilarity curve. (a) D = 0.23, p = 0.90 for the Bray-Curtis curve; (b) D = 0.38, p = 0.30 for the Simpson dissimilarity curve.
Extended Data Fig. 5 Autocorrelation function plots for all variables for which correlations were tested.
Autocorrelation function plots for variables tested for correlation in this study. No variable shows significant autocorrelation, which would be indicated by columns passing the dashed blue horizontal lines at lags greater than 0.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Rights and permissions
About this article
Cite this article
Penny, A., Kröger, B. Impacts of spatial and environmental differentiation on early Palaeozoic marine biodiversity. Nat Ecol Evol 3, 1655–1660 (2019). https://doi.org/10.1038/s41559-019-1035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-019-1035-7