Abstract
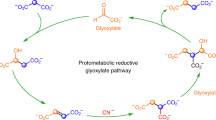
Autotrophic theories for the origin of life propose that CO2 was the carbon source for primordial biosynthesis. Among the six known CO2 fixation pathways in nature, the acetyl-CoA (AcCoA; or Wood–Ljungdahl) pathway is the most ancient, and relies on transition metals for catalysis. Modern microbes that use the AcCoA pathway typically fix CO2 with electrons from H2, which requires complex flavin-based electron bifurcation. This presents a paradox: how could primitive metabolic systems have fixed CO2 before the origin of proteins? Here, we show that native transition metals (Fe0, Ni0 and Co0) selectively reduce CO2 to acetate and pyruvate—the intermediates and end-products of the AcCoA pathway—in near millimolar concentrations in water over hours to days using 1–40 bar CO2 and at temperatures from 30 to 100 °C. Geochemical CO2 fixation from native metals could have supplied critical C2 and C3 metabolites before the emergence of enzymes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ruiz-Mirazo, K., Briones, C. & de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366 (2014).
Peretó, J. Out of fuzzy chemistry: from prebiotic chemistry to metabolic networks. Chem. Soc. Rev. 41, 5394–5403 (2012).
Sutherland, J. D. Studies on the origin of life—the end of the beginning. Nat. Rev. Chem. 1, 0012 (2017).
Berg et al. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 8, 447–460 (2010).
Hügler, M. & Sievert, S. M. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annu. Rev. Mar. Sci. 3, 261–289 (2011).
Ljungdahl, L. G., Irion, E. & Wood, H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and co-(methyl)-5-methoxy-benzimidizolycobamide as intermediates with Clostridium thermoaceticum. Biochemistry 4, 2771–2779 (1965).
Fuchs, G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658 (2011).
Weiss, M. C. et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 (2016).
Can, M., Armstrong, F. A. & Ragsdale, S. W. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase and acetyl-CoA synthase. Chem. Rev. 114, 4149–4174 (2014).
Schwarz, G., Mendel, R. R. & Ribbe, M. W. Molybdenum cofactors, enzymes and pathways. Nature 460, 839–847 (2009).
Schuchmann, K. & Müller, V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014).
Furdui, C. & Ragsdale, S. W. The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood–Ljungdahl pathway. J. Biol. Chem. 275, 28494–28499 (2000).
Martin, W. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. B 362, 1887–1926 (2007).
Herrmann, G., Jayamani, E., Mai, G. & Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 190, 784–791 (2008).
Huber, C. & Wächtershäuser, G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–247 (1997).
Cody, G. D. et al. Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1340 (2000).
Sousa, F. L., Preiner, M. & Martin, W. F. Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr. Opin. Microbiol. 43, 77–83 (2018).
Kato, S., Yumoto, I. & Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Env. Microbiol. 81, 67–73 (2015).
Daniels, L., Belay, N., Rajagopal, B. S. & Weimer, P. J. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237, 509–511 (1987).
Muchowska, K. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017).
He, C., Tian, G., Liu, Z. & Feng, S. A mild hydrothermal route to fix carbon dioxide to simple carboxylic acids. Org. Lett. 12, 649–651 (2010).
Sousa, F. & Martin, W. F. Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon metabolism. Biochim. Biophys. Acta 1837, 964–981 (2014).
Morowitz, H. J., Srinivasan, V. & Smith, E. Ligand field theory and the origin of life as an emergent feature of the periodic table of elements. Biol. Bull. 219, 1–6 (2010).
Camprubi, E., Jordan, S. F., Vasiliadou, R. & Lane, N. Iron catalysis at the origin of life. IUBMB Life 69, 373–381 (2017).
Moore, E. K., Jelen, B. I., Giovanelli, D., Raanan, H. & Falkowski, P. G. Metal availability and the expanding network of microbial metabolisms in the Archaean eon. Nat. Geosci. 10, 629–636 (2017).
Guan, G. et al. Reduction of aqueous CO2 at ambient temperature using zero-valent iron-based composites. Green Chem. 5, 630–634 (2003).
Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Evans, M. C. W., Buchanan, B. B. & Arnon, D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl Acad. Sci. USA 55, 928–934 (1966).
Wächtershäuser, G. Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 52, 452–484 (1988).
Smith, E. & Morowitz, H. J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere (Cambridge Univ. Press, Cambridge, 2016).
Morowitz, H. J., Kostelnik, J. D., Yang, J. & Cody, G. D. The origin of intermediary metabolism. Proc. Natl Acad. Sci. USA 97, 7704–7708 (2000).
Smith, E. & Morowitz, H. J. Universality in intermediary metabolism. Proc. Natl Acad. Sci. USA 101, 13168–13173 (2004).
Braakman, R. & Smith, E. The emergence and early evolution of biological carbon-fixation. PLoS Comp. Biol. 8, e1002455 (2012).
Braakman, R. & Smith, E. The compositional and evolutionary logic of metabolism. Phys. Biol. 10, 011001 (2013).
Chandru, K., Gilbert, A., Butch, C., Aono, M. & Cleaves, H. J. The abiotic chemistry of thiolated acetate derivatives and the origin of life. Sci. Rep. 6, 29883 (2016).
Roldan, A. et al. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem. Commun. 51, 7501–7504 (2015).
Klöck, W., Palme, H. & Tobschall, H. J. Trace elements in natural metallic iron from Disko Island, Greenland. Contrib. Mineral. Petrol. 93, 273–282 (1986).
McCollom, T. M. Abiotic methane formation during experimental serpentinization of olivine. Proc. Natl Acad. Sci. USA 113, 13965–13970 (2016).
Sleep, N. H., Meibom, A., Fridriksson, T, Coleman, R. G. & Bird, D. K. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci. USA 101, 12818–12823 (2004).
Frost, D. J. et al. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 428, 409–412 (2004).
Darling, D. J. The Universal Book of Astronomy 260 (Wiley, Hoboken, 2004).
Krot, A. N., Keil, K., Scott, E. R. D., Goodrich, C. A. & Weisberg, M. K. in Treatise on Geochemistry 2nd edn Vol. 1 (eds Holland, H. & Turekian, K.) 1–63 (Elsevier, Oxford, 2014).
Russell, M. J., Hall, A. J. & Mellersh, A. R. in Natural and Laboratory Simulated Thermal Geochemical Processes (ed. Ikan, R.) 325–388 (Springer, Dordrecht, 2003).
Bassez, M.-P. Water, air, earth and cosmic radiation. Orig. Life Evol. Biosph. 45, 5–13 (2015).
Bassez, M.-P. Anoxic and oxic oxidation of rocks containing Fe(II)Mg-silicates and Fe(II)-monosulfides as source of Fe(III)-minerals and hydrogen. Geobiotropy. Orig. Life Evol. Biosph. 47, 453–480 (2017).
Cooper, G., Reed, C., Nguyen, D., Carter, M. & Wang, Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc. Natl Acad. Sci. USA 108, 14015–14020 (2011).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic earth. PLoS Biol. 6, e18 (2008).
Acknowledgements
This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement 639170). Further funding was provided by a grant from LabEx ‘Chemistry of Complex Systems’. L. Allouche, M. Coppe and B. Vincent are gratefully acknowledged for assistance with the NMR experiments. We thank E. Smith and W. F. Martin for critical readings of this manuscript.
Author information
Authors and Affiliations
Contributions
J.M. supervised the research and the other authors performed the experiments. All authors contributed intellectually throughout the study. J.M. and K.B.M wrote the paper, and S.J.V. and K.B.M. assembled the Supplementary Information. Important preliminary experiments were carried out by P.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–35, Supplementary Tables 1–19
Rights and permissions
About this article
Cite this article
Varma, S.J., Muchowska, K.B., Chatelain, P. et al. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat Ecol Evol 2, 1019–1024 (2018). https://doi.org/10.1038/s41559-018-0542-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0542-2
This article is cited by
-
Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents
Communications Earth & Environment (2024)
-
Primitive purine biosynthesis connects ancient geochemistry to modern metabolism
Nature Ecology & Evolution (2024)
-
Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases
Archives of Toxicology (2024)
-
Methane formation driven by light and heat prior to the origin of life and beyond
Nature Communications (2023)
-
Ambient temperature CO2 fixation to pyruvate and subsequently to citramalate over iron and nickel nanoparticles
Nature Communications (2023)