Abstract
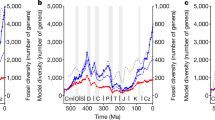
The end of the Pliocene marked the beginning of a period of great climatic variability and sea-level oscillations. Here, based on a new analysis of the fossil record, we identify a previously unrecognized extinction event among marine megafauna (mammals, seabirds, turtles and sharks) during this time, with extinction rates three times higher than in the rest of the Cenozoic, and with 36% of Pliocene genera failing to survive into the Pleistocene. To gauge the potential consequences of this event for ecosystem functioning, we evaluate its impacts on functional diversity, focusing on the 86% of the megafauna genera that are associated with coastal habitats. Seven (14%) coastal functional entities (unique trait combinations) disappeared, along with 17% of functional richness (volume of the functional space). The origination of new genera during the Pleistocene created new functional entities and contributed to a functional shift of 21%, but minimally compensated for the functional space lost. Reconstructions show that from the late Pliocene onwards, the global area of the neritic zone significantly diminished and exhibited amplified fluctuations. We hypothesize that the abrupt loss of productive coastal habitats, potentially acting alongside oceanographic alterations, was a key extinction driver. The importance of area loss is supported by model analyses showing that animals with high energy requirements (homeotherms) were more susceptible to extinction. The extinction event we uncover here demonstrates that marine megafauna were more vulnerable to global environmental changes in the recent geological past than previously thought.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brook, B. W., Sodhi, N. S. & Bradshaw, C. J. A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (2008).
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 (2015).
Harnik, P. G. et al. Extinctions in ancient and modern seas. Trends Ecol. Evol. 27, 608–617 (2012).
Miller, K. G. et al. The phanerozoic record of global sea-level change. Science 310, 1293–1298 (2005).
De Boer, B., van de Wal, R. S. W., Bintanja, R., Lourens, L. J. & Tuenter, E. Cenozoic global ice-volume and temperature simulations with 1-D ice-sheet models forced by benthic delta O-18 records. Ann. Glaciol. 51, 23–33 (2010).
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Lisiecki, L. E. & Raymo, M. E. Plio–Pleistocene climate evolution: trends and transitions in glacial cycle dynamics. Quat. Sci. Rev 26, 56–69 (2007).
Van Woesik, R. et al. Hosts of the plio-pleistocene past reflect modern-day coral vulnerability. Proc. R. Soc. Lond. B Biol. Sci. 279, 2448–2456 (2012).
Valentine, J. W. & Jablonski, D. Biotic effects of sea-level change: the pleistocene test. J. Geophys. Res. Solid Earth 96, 6873–6878 (1991).
Lewison, R. L., Crowder, L. B., Read, A. J. & Freeman, S. A. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604 (2004).
McClain, C. R. et al. Sizing ocean giants: patterns of intraspecific size variation in marine megafauna. PeerJ 3, e715 (2015).
Marx, F. G. & Uhen, M. D. Climate, critters, and cetaceans: Cenozoic drivers of the evolution of modern whales. Science 327, 993–996 (2010).
Pyenson, N. D. & Sponberg, S. N. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. J. Mamm. Evol. 18, 269–288 (2011).
Uhen, M. D. & Pyenson, N. D. Diversity estimates, biases, and historiographic effects: resolving cetacean diversity in the tertiary. Palaeontol. Electronica 10, 1–22 (2007).
Boessenecker, R. W. Pleistocene survival of an archaic dwarf baleen whale (Mysticeti: Cetotheriidae). Naturwissenschaften 100, 365–71 (2013).
Ando, T. & Fordyce, R. E. Evolutionary drivers for flightless, wing-propelled divers in the Northern and Southern hemispheres. Palaeogeogr. Palaeoclimatol. Palaeoecol. 400, 50–61 (2014).
Olson, S. L. An early pliocene marine avifauna from Duinefontein Cape Province South Africa. Ann. S. Afr. Mus. 95, 147–164 (1985).
Sorbi, S., Domning, D. P., Vaiani, S. C. & Bianucci, G. Metaxytherium subapenninum (Bruno, 1839) (Mammalia, Dugongidae), the latest sirenian of the mediterranean basin. J. Vert. Paleontol. 32, 686–707 (2012).
Velez-Juarbe, J., Domning, D. P. & Pyenson, N. D. Iterative evolution of sympatric seacow (Dugongidae, Sirenia) assemblages during the past similar to 26 million years. PloS ONE 7, e31294 (2012).
Domning, D. P. Sirenians, seagrasses, and Cenozoic ecological change in the Caribbean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 166, 27–50 (2001).
Pimiento, C. & Balk, M. A. Body-size trends of the extinct giant shark Carcharocles megalodon: a deep-time perspective on marine apex predators. Paleobiology 41, 479–490 (2015).
Pimiento, C. & Clements, C. F. When did Carcharocles megalodon become extinct? A new analysis of the fossil record. PloS ONE 9, e111086 (2014).
Dodd, C. K. & Morgan, G. S. Fossil sea-turtles from the early Pliocene Bone Valley Formation, Central Florida. J. Herpetol. 26, 1–8 (1992).
Leonard-Pingel, J. S., Jackson, J. B. C. & O’Dea, A. Changes in bivalve functional and assemblage ecology in response to environmental change in the Caribbean Neogene. Paleobiology 38, 509–524 (2012).
Estes, J. A. et al. Trophic downgrading of planet Earth. Science 333, 301–306 (2011).
Doughty, C. E. et al. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. USA 113, 868–873 (2016).
Malhi, Y. et al. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl Acad. Sci. USA 113, 838–846 (2016).
Boessenecker, R. W. A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: pinnipeds and cetaceans. Geodiversitas 35, 815–940 (2012).
Villeger, S., Novack-Gottshall, P. M. & Mouillot, D. The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol. Lett. 14, 561–568 (2011).
Raup, D. M. & Sepkoski, J. J. Jr Mass extinctions in the marine fossil record. Science 215, 1501–1503 (1982).
Bambach, R. K., Bush, A. M. & Erwin, D. H. Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1–22 (2007).
Mouillot, D., Graham, N. A. J., Villeger, S., Mason, N. W. H. & Bellwood, D. R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177 (2013).
Aberhan, M. & Kiessling, W. Persistent ecological shifts in marine molluscan assemblages across the end-Cretaceous mass extinction. Proc. Natl Acad. Sci. USA 112, 7207–7212 (2015).
Foster, W. J. & Twitchett, R. J. Functional diversity of marine ecosystems after the Late Permian mass extinction event. Nat. Geosci. 7, 233–238 (2014).
Dineen, A. A., Fraiser, M. L. & Sheehan, P. M. Quantifying functional diversity in pre- and post-extinction paleocommunities: a test of ecological restructuring after the end-Permian mass extinction. Earth Sci. Rev. 136, 339–349 (2014).
Duffy, J. E., Lefcheck, J. S., Stuart-Smith, R. D., Navarrete, S. A. & Edgar, G. J. Biodiversity enhances reef fish biomass and resistance to climate change. Proc. Natl Acad. Sci. USA 113, 6230–6235 (2016).
Dick, D. G. & Maxwell, E. E. The evolution and extinction of the ichthyosaurs from the perspective of quantitative ecospace modelling. Biol. Lett. 11, 20150339 (2015).
Lawton, J. H. What do species do in ecosystems. Oikos 71, 367–374 (1994).
Altieri, A. H. et al. Tropical dead zones and mass mortalities on coral reefs. Proc. Natl Acad. Sci. USA 114, 3660–3665 (2017).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Wernberg, T. et al. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172 (2016).
Silvestro, D., Schnitzler, J., Liow, L. H., Antonelli, A. & Salamin, N. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Syst. Biol. 63, 349–367 (2014).
Villafaña, J. A. & Rivadeneira, M. M. Rise and fall in diversity of Neogene marine vertebrates on the temperate Pacific coast of South America. Paleobiology 40, 659–674 (2014).
Finnegan, S. et al. Paleontological baselines for evaluating extinction risk in the modern oceans. Science 348, 567–570 (2015).
Mouillot, D. et al. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13757–13762 (2014).
Coux, C., Rader, R., Bartomeus, I. & M., T. J. Linking species functional roles to their network roles. Ecol. Lett. 19, 762–770 (2016).
Lefcheck, J. S. & Duffy, J. E. Multitrophic functional diversity predicts ecosystem functioning in experimental assemblages of estuarine consumers. Ecology 96, 2973–2983 (2015).
Dehling, D. M., Jordano, P., Schaefer, H. M., Boehning-Gaese, K. & Schleuning, M. Morphology predicts species’ functional roles and their degree of specialization in plant–frugivore interactions. Proc. R. Soc. Lond. B Biol. Sci. 283, 20152444 (2016).
Cadotte, M. W., Carscadden, K. & Mirotchnick, N. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087 (2011).
Kozlowski, J. & Gawelczyk, A. T. Why are species’ body size distributions usually skewed to the right? Funct. Ecol. 16, 419–432 (2002).
Duffy, J. E. Biodiversity loss, trophic skew and ecosystem functioning. Ecol. Lett. 6, 680–687 (2003).
Duffy, J. E. Biodiversity and ecosystem function: the consumer connection. Oikos 99, 201–219 (2002).
Holland, S. M. Sea level change and the area of shallow-marine habitat: implications for marine biodiversity. Paleobiology 38, 205–217 (2012).
Jackson, J. B. C., Jung, P., Coates, A. G. & Collins, L. S. Diversity and extinction of tropical American mollusks and emergence of the isthmus of Panama. Science 260, 1624–1626 (1993).
Budd, A. F., Johnson, K. G. & Stemann, T. A. in Evolution and Environment in Tropical America (eds Jackson, B. C., Budd, A. F. & Coates, A. G.) 168–204 (Univ. Chicago Press, 1996).
Allmon, W. D., Emslie, S. D., Jones, D. S. & Morgan, G. S. Late Neogene oceanographic change along Florida’s west coast: evidence and mechanisms. J. Geol. 104, 143–162 (1996).
Klaus, J. S. et al. Rise and fall of Pliocene free-living corals in the Caribbean. Geology 39, 375–378 (2011).
Todd, J. A. et al. The ecology of extinction: molluscan feeding and faunal turnover in the Caribbean Neogene. Proc. R. Soc. Lond. B Biol. Sci. 269, 571–577 (2002).
O’Dea, A. et al. Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl Acad. Sci. USA 104, 5501–5506 (2007).
Smith, J. T. & Jackson, J. B. C. Ecology of extreme faunal turnover of tropical American scallops. Paleobiology 35, 77–93 (2009).
Valenzuela-Toro, A. M., Gutstein, C. S., Varas-Malca, R. M., Suarez, M. E. & Pyenson, N. D. Pinniped turnover in the South Pacific Ocean: new evidence from the Plio−Pleistocene of the Atacama Desert, Chile. J. Vert. Paleontol. 33, 216–223 (2013).
Metcalf, J. L. et al. Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the last deglaciation. Sci. Adv. 2, e1501682 (2016).
Pimiento, C. et al. Geographical distribution patterns of Carcharocles megalodon over time reveal clues about extinction mechanisms. J. Biogeogr. 43, 1645–1655 (2016).
McNab, B. K. Resources and energetics determined dinosaur maximal size. Proc. Natl Acad. Sci. USA 106, 12184–12188 (2009).
Grady, J. M., Enquist, B. J., Dettweiler-Robinson, E., Wright, N. A. & Smith, F. A. Evidence for mesothermy in dinosaurs. Science 344, 1268–1272 (2014).
Payne, J. L., Bush, A. M., Heim, N. A., Knope, M. L. & McCauley, D. J. Ecological selectivity of the emerging mass extinction in the oceans. Science 14, aaf2416 (2016).
Boyles, J. G., Seebacher, F., Smit, B. & McKechnie, A. E. Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690 (2011).
McNab, B. K. Energetics, body size, and the limits to endothermy. J. Zool. 199, 1–29 (1983).
Wright, D. H. Species-energy theory: an extension of species-area theory. Oikos 1, 496–506 (1983).
Pyenson, N. D. & Lindberg, D. R. What happened to grey whales during the Pleistocene? The ecological impact of sea level change on benthic feeding areas in the North Pacific Ocean. PloS ONE 6, e21295 (2011).
Davidson, A. D. et al. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl Acad. Sci. USA 109, 3395–3400 (2012).
Wilmers, C. C., Estes, J. A., Edwards, M., Laidre, K. L. & Konar, B. Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front Ecol. Environ. 10, 409–415 (2012).
Gradstein, F. M., Ogg, G. & Schmitz, M. The Geologic Time Scale 2012 (Elsevier, 2012).
Silvestro, D., Salamin, N. & Schnitzler, J. PyRate: a new program to estimate speciation and extinction rates from incomplete fossil data. Methods Ecol. Evol. 5, 1126–1131 (2014).
Silvestro, D., Cascales-Miñana, B., Bacon, C. D. & Antonelli, A. Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436 (2015).
Hoeting, J. A., Madigan, D., Raftery, A. E. & Volinsky, C. T. Bayesian model averaging: a tutorial. Stat. Sci. 14, 382–417 (1999).
Gibbard, P. L., Head, M. J. & Walker, M. J. Formal ratification of the Quaternary system/period and the Pleistocene series/epoch with a base at 2.58 Ma. J. Quat. Sci 25, 96–102 (2010).
Laliberté, E. & Shipley, B. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-11 (2011).
Maire, E., Grenouillet, G., Brosse, S. & Villeger, S. How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob. Ecol. Biogeogr. 24, 728–740 (2015).
Kowalewski, M. I. & Novack-Gottshall, P. H. in Quantitative Methods in Paleobiology (eds Alroy, J. & Hunt, G.) 19–54 (The Paleontological Society, 2010).
Foote, M. Origination and extinction components of taxonomic diversity: general problems. Paleobiology 26, 74–102 (2000).
Amante, C. & Eakins, B. W. 2009 ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis (NOAA Technical Memorandum NESDIS NGDC-24, National Geophysical Data Center, accessed June 2017).
Hijmans, R. J. et al. Package ‘raster’. R package version 2.5-8 (2015).
Bates, D. & Martin, M. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Walsh, C., Mac Nally, R. & Walsh, M. C. The hier. part package. R package version 1.4-0 (2003).
Acknowledgements
We thank M. Sánchez-Villagra for his support during the development of this research, S. Villegér, A. Antonelli, F. Leprieur, J. Lefcheck and L. Gamfeldt for their valuable suggestions, C. Ricotta and K. Boersma for their assistance with the use of R functions, B. Mcnab and M. Balk for their insights on thermoregulation, and J. Velez-Juarbe for his support assigning traits to marine mammals. We are grateful for the constructive comments provided by P. Novack-Gottshall, which significantly improved this work. PyRate analyses were run at the high-performance computing centre Vital-IT of the Swiss Institute of Bioinformatics (Lausanne, Switzerland). C.P. was supported by a Forschungskredit postdoctoral fellowship from the University of Zurich (FK-15-105), J.N.G. was supported by a European Union Marie Curie Career Integration Grant (FP7 MC CIG 61893), D.S. was funded by the Swedish Research Council (2015-04748) and S.V. was first supported by the Universidad de Alcalá postdoctoral programme, and then by the Alexander von Humboldt Foundation and the Federal Ministry for Education and Research (Germany). This is the Paleobiology Database publication number 284.
Author information
Authors and Affiliations
Contributions
C.P., J.N.G. and C.J. designed the research, C.P., J.N.G. and M.D.U. performed the research, C.P., J.N.G., C.F.C., D.S., S.V. and M.D.U. analysed the data, C.P. and J.N.G. wrote the paper, and C.F.C., S.V., D.S., M.D.U. and C.J. improved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Methods, 9 Supplementary Tables, 12 Supplementary Figures
Supplementary Dataset 1
Species included in the analysis with details on class, maximum body size, guild, vertical position, habitat, metabolic control and status
Supplementary Dataset 2
References supporting marine megafaunal occurrences
Rights and permissions
About this article
Cite this article
Pimiento, C., Griffin, J.N., Clements, C.F. et al. The Pliocene marine megafauna extinction and its impact on functional diversity. Nat Ecol Evol 1, 1100–1106 (2017). https://doi.org/10.1038/s41559-017-0223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-017-0223-6
This article is cited by
-
Investigating the Relationship Between Body Shape and Life History Traits in Toothed Whales: Can Body Shape Predict Fast-Slow Life Histories?
Evolutionary Biology (2023)
-
Fossil data support a pre-Cretaceous origin of flowering plants
Nature Ecology & Evolution (2021)
-
Late Neogene evolution of the Peruvian margin and its ecosystems: a synthesis from the Sacaco record
International Journal of Earth Sciences (2021)
-
Remarkable multicuspid teeth in a new elusive skate (Chondrichthyes, Rajiformes) from the Mediterranean Pliocene
PalZ (2021)
-
Impacts of speciation and extinction measured by an evolutionary decay clock
Nature (2020)