Abstract
The terminal Pleistocene/Holocene boundary (approximately 12–8 thousand years ago) represented a major ecological threshold for humans, both as a significant climate transition and due to the emergence of agriculture around this time. In the highlands of New Guinea, climatic and environmental changes across this period have been highlighted as potential drivers of one of the earliest domestication processes in the world. We present a terminal Pleistocene/Holocene palaeoenvironmental record (12–0 thousand years ago ) of carbon and oxygen isotopes in small mammal tooth enamel from the site of Kiowa. The results show that tropical highland forest and open mosaics, and the human subsistence focused on these environments, remained stable throughout the period in which agriculture emerged at nearby Kuk Swamp. This suggests the persistence of tropical forest foraging among highland New Guinea groups and highlights that agriculture in the region was not adopted as a unilinear or dramatic, forced event but was locally and historically contingent.
Similar content being viewed by others
The transition from the terminal Pleistocene to the Holocene witnessed increasingly intensive human manipulation of plant and animal resources that resulted in genetic and phenotypic changes in various species as part of what has been termed the ‘origins of agriculture’1. This process has been cited as one of the most significant ecological moments in human evolutionary history2,3, representing a shift in human interactions with the natural world that was to have global environmental ramifications4,5. The emergence of this new form of subsistence has elsewhere been linked to dramatic climatic and environmental processes witnessed across the terminal Pleistocene/Holocene transition and into the Holocene6,7, yet the horticulture-style cultivation seen in the tropics is often ignored in such discussions. This is despite the fact that active human manipulation of plants and animals, including deliberate anthropogenic burning of forests to encourage plant growth8,9 and the deliberate translocation of small mammals10, occurred in tropical forest environments as early as 45 thousand years ago (ka) and 20 ka, respectively.
It is now evident that one of the clearest and earliest examples of the mutualistic relationship between humans and their plant-foods comes from Kuk Swamp in the tropical highlands of New Guinea1,11,12. Here, in a montane tropical rainforest and grassland ecotone, terminal Pleistocene human foragers moved and tended the tropical plants of yam (Dioscorea sp.), banana (Musa sp.) and taro (Colocasia sp.) taxa until these species were fully ‘domesticated’ during the Early–Middle Holocene1,11,13. Although decades of research has described the archaeological sequence of Kuk Swamp, little is known of foraging behaviour elsewhere in the New Guinea Highlands and the drivers behind the intensification of tropical plant manipulation at this time. As in other regions, it has been suggested that this process was stimulated by climatic fluctuation across the Pleistocene/Holocene boundary1,14. However, there are currently no detailed, well-dated, ‘on-site’ palaeoenvironmental records, directly associated with forager behaviour, in this region to support or refute such hypotheses.
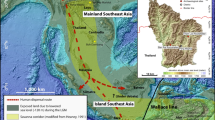
We applied stable carbon and oxygen isotope analysis to small mammal tooth enamel from the archaeological site of Kiowa (Supplementary Text 1 and Supplementary Tables 1 and 2), which is located at a similar ecological and altitudinal position to Kuk Swamp in the Central Highlands of New Guinea (Figs 1 and 2), to produce a well-dated, palaeoenvironmental sequence associated with terminal Pleistocene/Holocene forager behaviour (12–0 ka) (Supplementary Table 1)15–17. Throughout the sequence, large core tools and small retouched flakes were found associated with a large faunal assemblage dominated by fruit bats (Aproteles bulmerae), cuscus (Phalangeridae), ringtailed possum (Pseudocheiridae) and macropods (Macropodidae) (Supplementary Texts 1 and 2). Unlike at Kuk Swamp, there is no evidence for local landscape modification or a shift towards plant cultivation during the Holocene, with the presence of these fauna remaining stable throughout the duration of site occupation (Supplementary Text 1).
Isotope analysis of archaeological small mammalian fauna such as those identified at Kiowa is an increasingly popular method of local palaeoenvironmental reconstruction due to their high frequency (and therefore large sample sizes), small home ranges and high habitat discrimination18,19. Stable carbon isotopes of mammalian faunal enamel primarily reflect the proportions of C3 and C4 biomass in the diet in the tropics and subtropics; low faunal δ13C indicates reliance on 13C-depleted C3 resources relative to 13C-enriched C4 resources available in open areas20–22. In dense C3 forest, very low δ13C values for C3 plants are attributed to the ‘canopy effect’, in which vegetation growing under closed forest canopy is markedly 13C-depleted due to low light23 and recycled CO2 (ref. 24). These environmental factors can be tracked into mammalian tooth enamel, following an established dietary fractionation factor20,25 of between approximately 12‰ and 14‰. Tooth enamel δ18O values primarily reflect water consumed, either as drinking water or plant water, as well as some input from bound oxygen in food26,27. In the tropics, imbibed water δ18O will be dictated by the amount of rainfall28 or evapotranspiration in plants that, in turn, can be associated with forest coverage. Tropical forests are thought to have retreated during cooler glacial periods when the atmospheric CO2 partial pressure (pCO2) was low, giving way to more open C3/C4 grasslands and shrublands, particularly at higher altitudes29,30, with corresponding impacts on faunal diets and ingested water.
Results
The δ13C and δ18O results for all small mammal faunal groups (cuscus, ringtail possum, bat and macropod) from the site of Kiowa are shown in Fig. 3 and Supplementary Table 3.
The δ13C values covered a wide range (−14.5‰ to −8.7‰). Based on a comparison with stable isotope analysis of modern faunal tooth enamel (corrected for the fossil fuel effect31), the data showed an environmental range from closed forest environments (≤−14.0‰) to open-woodland mosaic C3 habitats (approximately −12.0‰ to −9.0‰)21,22,32,33. This range fits with the modern ecological preferences of the faunal groups sampled (Supplementary Text 2). The δ13C range remained broadly consistent across the terminal Pleistocene and Holocene levels at the site with some elements of dense forest habitat and more open C3 environments present throughout the period of human occupation. Analysis of variance (ANOVA) testing indicated that there was no difference in overall faunal δ13C values between the levels (F(6,130) = 1.61, P > 0.05) (Supplementary Table 4).
The δ18O data from the Kiowa fauna showed a similarly large range (−12.6‰ to −6.4‰). Low δ18O is expected in the tropics as a result of the high intensity of rainfall28. All of the fauna analysed were obligate drinkers and therefore this range is probably indicative of variability in precipitation values, in open drinking water sources in the vicinity of the site, and in food. The faunal groups sampled had varying reliance on open water sources, while; cuscus, ringtail possums and bats are known to drink nocturnally, which probably contributed to the size of this range (Supplementary Text 2). As with δ13C, the δ18O values and range also remained consistent across the terminal Pleistocene and Holocene periods of site activity, suggesting little change in the influences that governed hydrological isotope values in the tropics during human occupation at the site19,34,35. An ANOVA test demonstrated that there was no significant difference between overall fauna δ18O values from the different levels (F(6,130) = 1.008, P > 0.05) (Supplementary Table 5).
There were, however, consistent δ13C distinctions evident between the faunal groups sampled (F(3,130) = 30.79, P < 0.05; ANOVA) (Supplementary Table 4). When post hoc Tukey pairwise testing was applied, statistically significant differences were found between macropods and bats, ringtail possums and bats, macropods and cuscus, and ringtail possums and cuscus (Supplementary Table 6). Bat and cuscus δ13C values were at the higher end of the δ13C range, relative to the ringtail possums, while macropods spanned the whole range (Supplementary Fig. 3). As bats are known to travel large distances to obtain food36,37, the contribution of resources from open C3 environments is unsurprising. The distinction between the cuscus and both the ringtail possums and macropods may suggest that cuscus could survive in more open forest mosaics than these other taxa (Supplementary Text 2). ANOVA testing also demonstrated differences in δ18O values between the groups of taxa sampled (F(3,130) = 6.015, P < 0.05; Supplementary Table 5). When post hoc Tukey pairwise testing was applied, statistically significant differences were observed between macropods and bats, and ringtail possums and macropods (Supplementary Table 7). These differences may be linked to the nocturnal habits of bats and ringtail possums, compared with the diurnal feeding and drinking habits of macropods (Supplementary Text 2).
There was no variation in δ13C and δ18O values between stratigraphic levels when ANOVA tests were performed on individual faunal groups (Fig. 4). Ringtail possums showed no variation in δ13C (F(6,28) = 0.860, P > 0.05; Supplementary Table 8) or δ18O (F(6,28) = 1.582, P > 0.05; Supplementary Table 9) by level. The same was true for cuscus (δ13C: F(6,28) = 0.738, P > 0.05, Supplementary Table 10; δ18O: F(6,28) = 2.127, P > 0.05, Supplementary Table 11) and bats (δ13C: F(6,29) = 1.568, P > 0.05, Supplementary Table 12; δ18O: F(6,29) = 1.273, P > 0.05, Supplementary Table 13). No variation in δ13C by level was found for macropods (F(6,27) = 0.710, P > 0.05; Supplementary Table 14). However, there was a significant difference in macropod δ18O by level (F(6,27) = 2.636, P < 0.05; Supplementary Table 15). This may be a result of macropods feeding and drinking diurnally, and therefore more strongly reflecting palaeoenvironmental evapotranspiration-linked changes in δ18O, compared with the other taxa sampled. That said, when post hoc Tukey pairwise testing was applied, no specific inter-level δ18O differences were found for the taxon group (Supplementary Table 16).
Discussion
Overall, the results indicate stability in faunal diets and drinking water sources, and their associated environments, from the terminal Pleistocene into the Holocene in the vicinity of Kiowa. The data presented here are consistent with other palaeoenvironmental evidence from South Asia38,39, Southeast Asia9,40 and the New Guinea Highlands themselves41 that suggest the persistence of tropical forests, albeit of varying structure, in this part of the world between 12 and 0 ka. Significant, rapid climate fluctuations, particularly in the form of submontane tropical forest encroachment under the warmer conditions of the Holocene, have been argued for in the highlands of New Guinea across the terminal Pleistocene boundary42–44, and have been suggested to coincide with a peak in human forest burning around Kuk Swamp approximately 10–9 ka41,45. However, major changes in vegetation do not appear to have occurred in the vicinity of Kiowa, or at least not significantly enough to alter the isotope values in typically hunted prey. Since an ecotone of tropical forest and open C3 environments remains present within the hunting range of Kiowa’s human occupants, it seems unlikely that palaeoclimatic and palaeoenvironmental change drove the intensification of plant use and landscape modification witnessed at nearby Kuk Swamp during this period.
The lack of marked climate and environmental shifts in our data suggest that plant manipulation and domestication at Kuk Swamp is best seen as active anthropogenic modification of the landscape, rather than an environmental response. It could be argued that other reasons, including demographic change or an increase in social network densities46,47, should be used to explain one of the earliest global experiments with ‘agriculture’. However, given the paucity of evidence elsewhere in the region for ‘domestication’ processes at this time15,48, it is perhaps best to see Kuk Swamp as part of a diversity of stable, successful tropical forest foraging adaptations in the region that extend back to 45 ka8,48, through the Last Glacial Maximum49,50 and into the Holocene1,13. Evidence for an increasingly intensive relationship between human foragers and tropical forests across the terminal Pleistocene/Holocene transition in Sahul includes an increase in burning to stimulate plant growth51 and the deliberate translocation of animal protein between tropical forest islands10. Instead of a dramatic forced event, plant management at Kuk Swamp is part of the long-term relationships between hunter–gatherer communities and tropical forest habitats that have characterized this part of the world from the Late Pleistocene.
Tropical forests are currently undergoing a rehabilitation in the archaeological and anthropological literature as productive environments for human foraging52–54 and also for the development and spread of agricultural practices1,12,55,56. Yet, rather than reinforcing the familiar dichotomies of ‘hunter–gatherer’ and ‘farmer’, human subsistence strategies within these habitats challenge our perceptions of the unilinear movement of Homo sapiens towards agricultural production and sedentary populations57. Tropical forest foraging has apparently provided diverse and stable resources for humans in Melanesia since their arrival approximately 45 ka and through periods of climatic perturbation and the appearance of what might be termed ‘agriculture’ in certain localities11,55. Effective niche construction5 by human populations from 45 ka, including the burning of forest vegetation (perhaps to maintain ecotonal environments and promote useful plant growth) and the movement of animals between habitats, blur the lines between foragers and farmers. Our results show that the development of farming, in this region at least, was unlikely to have been sparked by climate change and is part of complex, long-term human modifications of the landscape and its wildlife that defy simplistic categories of ‘agricultural’ origins.
Methods
We sampled molar teeth from small mammal taxa that made up a consistently high portion of the mammalian assemblage throughout the archaeological sequence of Kiowa (Supplementary Text 1)15,58–60. Faunal remains were identified by Jim Menzies and David Bulmer using a modern reference collection housed in the Zoology Department at the University of Papua New Guinea. Molar teeth were taken from bats (Dobsonia magna), cuscus (Phalanger carmelitae), ringtail possums, (Pseudocheirus corinnae, P. cupreus and P. forbesi), and macropods (Dendrolagus and Thylogale). Where possible, all specimens were selected from the large East Extension, in levels directly associated with radiocarbon determinations to ensure secure stratigraphic control. Level designations followed ref. 15, with Levels 10A–B and 12B–12A grouped simply as Level 10 and 12, respectively. No fauna was sampled from Level 12C, which has been argued elsewhere as potentially representative of a natural accumulation of small mammal fauna by birds of prey, before human occupation (Supplementary Text 1)15.
The taxa sampled represented a diversity of environments, ecological niches and altitudinal zones (Supplementary Text 2), enabling a more detailed picture of local environmental change relevant to human hunters to be produced throughout the sequence. Cuscus (P. carmelitae) is a nocturnal inhabitant of primary tropical forest approximately 1,350–3,800 m above sea level (a.s.l.) and the possums (P. cupreus, P. corinnae and P. forbesi) are nocturnal and come mainly from undisturbed primary forest approximately 2,000 m a.s.l. and higher. The bat species sampled (D. magna) is abundant in tropical forest up to 2,700 m a.s.l. but is also known to range to savannah woodland at lower altitudes (Supplementary Text 2). Finally, the larger macropod individuals come from a diversity of forest habitats at different altitudes and include a mixture of nocturnal and diurnal feeders (Supplementary Text 2).
Despite primarily being nocturnal, these taxa are extensively documented in ethnographic and archaeological hunting assemblages60,61–66 and are found in association with human activity throughout the period of human occupation of Kiowa15. From Level 12B (12.4–11.7 ka), which contains the first material culture remains at Kiowa, the faunal assemblage notably changed from a broad array of small mammal species, to an increased frequency of large–medium sized bats, cuscus, ringtailed possums, macropods and some small–medium murids (Zones 1B–2B; ref. 60).
To avoid the nursing or weaning effect67, second and third molars were preferred as representing the ‘adult’ period of enamel formation and diet for bats, cuscus, ringtail possums, and macropods. Exterior surfaces of the teeth were cleaned using air-abrasion to remove any adhering external material. The roots of the molar teeth were removed and each tooth was sectioned to remove dirt from inside the tooth. Due to their small sizes, each tooth was crushed using an agate mortar and pestle19,35. An attempt was made to remove dentine from enamel where possible. However, as per ref. 19, given the relatively short formation times for these teeth, we assumed that dentine apatite and enamel apatite represented the same period and conditions.
All enamel powder was pretreated using a consistent technique to remove any organic or secondary carbonate contaminants68,69. This consisted of a series of washes in 1.5% sodium hypochlorite for 60 min, followed by three rinses in purified H2O and centrifugation, before 0.1 M acetic acid was added for 10 min, followed by another three rinses in purified H2O. Following reaction of the samples with 100% phosphoric acid, the gases evolved were analysed to identify the stable carbon and oxygen isotopic composition using a Gas Bench II (ThermoFisher) connected to a Delta V Advantage Mass Spectrometer (ThermoFisher). Carbon and oxygen isotope values were compared against an international standard registered by the International Atomic Energy Agency (NBS 19) and an in-house standard (MERCK). Replicate analysis of ostrich eggshell (OES) standards indicated that the machine measurement error was approximately ± 0.1‰ for δ13C and ± 0.2‰ for δ18O.
All δ13C and δ18O datasets were tested for normality using the Shapiro–Wilks test and histogram observations before statistical analysis. ANOVA tests were performed on faunal enamel δ13C and δ18O values from Kiowa to determine the influence of species and stratigraphic level on isotopic variation overall and for each faunal group (namely ringtail possums, cuscus, bats and macropods). Where variance was found to be significant, this was combined with a post hoc Tukey pairwise comparison to determine which taxa or stratigraphic levels were significantly different from each other, in terms of δ13C and δ18O values. Where data were non-normal a Kruskal–Wallis test was applied in addition to the parametric analyses described. In all cases, the results of the parametric and non-parametric analyses supported each other. We consequently only report the results of the ANOVA and, where relevant, post hoc Tukey pairwise comparison tests for consistency across the datasets. All statistical analyses were conducted using the software ‘R’70.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
Additional information
How to cite this article: Roberts, P., Gaffney, D., Lee-Thorp, J. & Summerhayes, G. Persistent foraging in the highlands of terminal Pleistocene/Holocene New Guinea. Nat. Ecol. Evol. 1, 0044 (2017).
References
Denham, T. P. et al. Origins of agriculture at Kuk Swamp in the highlands of New Guinea. Science 301, 189–193 (2003).
Bocquet-Appel, J. P. When the world’s population took off: the springboard of the Neolithic demographic transition. Science 333, 560–561 (2011).
Larson, G. et al. Current perspectives and the future of domestication studies. Proc. Natl Acad. Sci. USA 111, 6139–6146 (2014).
Fuller, D. Q. et al. The contribution of rice agriculture and livestock pastoralism to prehistoric methane levels: an archaeological assessment. Holocene 21, 743–759 (2011).
Boivin, N. et al. Ecological consequences of human niche construction: examining long-term anthropogenic shaping of global species distributions. Proc. Natl Acad. Sci. USA 113, 6388–6396 (2016).
Bar-Yosef, O. & Belfer-Cohen, A. The origins of sedentism and farming communities in the Levant. J. World Prehist. 3, 447–498 (1989).
Richerson, P. J., Boyd, R. & Bettinger, R. L. Was agriculture impossible during the Pleistocene but mandatory during the Holocene? A climate change hypothesis. Am. Antiquity 66, 387–411 (2001).
Summerhayes, G. R. et al. Human adaptation and plant use in Highland New Guinea 49,000 to 44,000 years ago. Science 330, 78–81 (2010).
Hunt, C. O., Gilbertson, D. D. & Rushworth, G. A 50,000-year record of late Pleistocene tropical vegetation and human impact in lowland Borneo. Quat. Sci. Rev. 37, 61–80 (2012).
Gosden, C. & Robertson, N. in Report of the Lapita Homeland Project (eds Allen, A. & Gosden, C.) 20–91 (Occasional Papers in Prehistory 20, Department of Prehistory, Research School of Pacific Studies, Australian National Univ., 1991).
Golson, J. in Sunda and Sahul: Prehistoric Studies in Southeast Asia, Melanesia and Australia (eds Allen, J. et al. ) 601–638 (Academic, 1977).
Harris, D. Early agriculture in New Guinea and the Torres Strait divide. Antiquity 69, 848–854 (1995).
Denham, T., Haberle, S. & Lentfer, C. New evidence and revised interpretations of early agriculture in Highland New Guinea. Antiquity 78, 839–857 (2004).
Haberle, S. G. & Lusty, A. C. Can climate influence cultural development? A view through time. Env. Hist. 6, 349–369 (2000).
Gaffney, D., Ford, A. & Summerhayes, G. Crossing the Pleistocene–Holocene transition in the New Guinea Highlands: evidence from the lithic assemblage of Kiowa rockshelter. J. Anthropol. Archaeol. 39, 223–246 (2015).
Gaffney, D., Ford, A. & Summerhayes, G. Sue Bulmer’s legacy in Highland New Guinea: a re-examination of the Bulmer Collection and future directions. Archaeol. Ocean 51, 23–32 (2016).
Denham, T. Revisiting the past: Sue Bulmer’s contribution to the archaeology of Papua New Guinea. Archaeol. Ocean 51, 5–10 (2016).
Grimes, S. T., Collinson, M. E., Hooker, J. J. & Mattey, D. P. Is small beautiful? A review of the advantages and limitations of using small mammal teeth and the direct laser fluorination analysis technique in the isotope reconstruction of past continental climate change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 208, 103–119 (2008).
Jeffrey, A., Denys, C., Stoetzel, E. & Lee-Thorp, J. A. Influences on the stable oxygen and carbon isotopes in gerbillid rodent teeth in semi-arid and arid environments: implications for past climate and environmental reconstruction. Earth Planet Sci. Lett. 428, 84–96 (2015).
Lee-Thorp, J. A. & van der Merwe, N. J. Carbon isotope analysis of fossil bone apatite. S. Afr. J. Sci. 83, 712–715 (1987).
Cerling, T. E., Hart, J. A. & Hart, T. B. Isotope ecology in the Ituri forest. Oecologia 138, 5–12 (2004).
Levin, N. E., Simpson, S. W., Quade, J., Cerling, T. E. & Frost, S. R. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. Geol. Soc. Am. Spec. Pap. 446, 215–234 (2008).
Farquhar, G. D., Ehleringer, J. R. & Hubick, K. T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537 (1989).
Van der Merwe, N. J. & Medina, E. The canopy effect, carbon isotope ratios and foodwebs in Amazonia. J. Archaeol. Sci. 18, 249–259 (1991).
Cerling, T. E. & Harris, J. M. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120, 247–363 (1999).
O’Grady, P. et al. Hydrogen and oxygen isotope ratios in body water and hair: modeling isotope dynamics in nonhuman primates. Am. J. Primatol. 74, 651–660 (2012).
Zazzo, A. et al. Isotopic composition of sheep wool records seasonality of climate and diet. Rapid Commun. Mass Spectrom. 29, 1357–1369 (2015).
Gonfiantini, R., Roche, M.-A., Olivry, J.-C., Fontes, J.-C. & Zuppi, G. M. The altitude effect on the isotopic composition of tropical rains. Chem. Geol. 181, 147–167 (2001).
Jolly, D. & Haxeltine, A. Effect of low glacial atmospheric CO2 on tropical African montane vegetation. Science 276, 786–788 (1997).
Mayle, F. E., Beerling, D. J., Gosling, W. D. & Bush, M. B. Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the Last Glacial Maximum. Phil. Trans. R. Soc. Lond. B 359, 499–514 (2004).
Francey, R. J. et al. A 1000-year high precision record of δ13C in atmospheric CO2 . Tellus B 51, 170–193 (1999).
Lee-Thorp, J. A., van der Merwe, N. J. & Brain, C. K. Isotopic evidence for dietary differences between two extinct baboon species from Swartkrans (South Africa). J. Hum. Evol. 18, 183–190 (1989).
Leichliter, J. N. et al. Small mammal insectivore stable carbon isotope compositions as habitat proxies in a South African savanna ecosystem. J. Archaeol. Sci. 8, 335–345 (2016).
D’Angela, D. & Longinelli, A. Oxygen isotopes in living mammal’s bone phosphate: further results. Chem. Geol. 86, 75–82 (1990).
Gehler, A., Tìtken, T. & Pack, A. Oxygen and carbon isotope variations in a modern rodent community—implications for palaeoenvironmental reconstructions. PLoS ONE 7, e49531 (2012).
Fleming, T. H., Nuñez, R. A. & Sternberg, L. S. L. Seasonal changes in the diets of migrant and non-migrant nectarivorous bats as revealed by carbon stable isotope analysis. Oecologia 94, 72–75 (1993).
Segers, J. L. & Broders, H. G. Carbon (δ13C) and nitrogen (δ15N) stable isotope signatures in bat fur indicate swarming sites have catchment areas for bats from different summering areas. PLoS ONE 10, e0125755 (2015).
Premathilake, R. & Risberg, J. Late Quaternary history of the Horton Plains, central Sri Lanka. Quat. Sci. Rev. 22, 1525–1541 (2003).
Roberts, P., et al. Direct evidence for human reliance on rainforest resources in late Pleistocene Sri Lanka. Science 347, 1246–1249 (2015).
Sun, X., Li, X., Luo, Y. & Chen, X. The vegetation and climate at the last glaciation on the emerged continental shelf of the South China Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 160, 301–316 (2000).
Hope, G. & Haberle, S. in Papuan Pasts: Studies in the Cultural, Linguistic and Biological History of the Papuan Speaking Peoples (eds Pawley, A. et al. ) 541–554 (Pacific Linguistics, Research School of Pacific and Asian Studies, Australian National Univ., 2005).
Haberle, S. G. Late Quaternary vegetation change in the Tari Basin, Papua New Guinea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 137, 1–24 (1998).
Haberle, S. G. The emergence of an agricultural landscape in the highlands of New Guinea. Archaeol. Ocean 38, 149–158 (2003).
Haberle, S. G., Hope, G. S. & van der Kaars, S. Biomass burning in Indonesia and Papua New Guinea: natural and human induced fire events in the fossil record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 171, 259–268 (2001).
Haberle, S. G., Lentfer, C., O’Donnell, S. & Denham, T. The palaeoenvironments of Kuk Swamp from the beginnings of agriculture in the highlands of Papua New Guinea. Quat. Int. 249, 129–139 (2012).
Binford, L. R. in New Perspectives in Archaeology (eds Binford, S. R. & Binford, L. R. ) 313–342 (Aldine, 1968).
Hayden, B. in Transitions to Agriculture in Prehistory (eds Gebauer, A. B. & Price, T. D. ) 11–18 (Prehistory, 1992).
Summerhayes, G. R., Field, J. H., Shaw, B. & Gaffney, D. The archaeology of forest exploitation and change in the tropics during the Pleistocene: the case of Northern Sahul (Pleistocene New Guinea). Quat. Int. http://dx.doi.org/10.1016/j.quaint.2016.04.023 (2016).
Pavlides, C. in A Pacific Odyssey: Archaeology and Anthropology in the Western Pacific. Papers in Honour of Jim Specht (eds Attenbrow, V. J. & Fullager, R. ) 97–108 (Australian Museum, 2004).
Leavesley, M. in Archaeology of Oceania: Australia and the Pacific Islands (ed. Lilley, I. ) 189–204 (Blackwell, 2006).
Fairbairn, A. S., Hope, G. S. & Summerhayes, G. R. Pleistocene occupation of New Guinea’s highland and subalpine environments. World Archaeol. 38, 371–386 (2006).
Mercader, J. (ed.) Under the Canopy: The Archaeology of Tropical Rainforests (Rutgers Univ. Press, 2002).
Roberts, P. & Petraglia, M. D. Pleistocene rainforests: barriers or attractive environments for early human foragers? World Archaeol. 47, 718–739 (2015).
Denham, T. P. in The Routledge Handbook of Bioarchaeology in Southeast Asia and the Pacific Islands (eds Oxenham, M. & Buckley, H. ) 409–425 (Routledge, 2016).
Bellwood, P. First Farmers: The Origins of Agricultural Societies (Wiley, 2004).
Denham, T. P. Early agriculture and plant domestication in New Guinea and Island Southeast Asia. Curr. Anthropol. 52, S379–S395 (2011).
Denham, T. P., Iriarte, I. & Vrydaghs, L. Rethinking Agriculture: Archaeological and Ethnoarchaeological Perspectives (Left Coast, 2007).
Bulmer, S. Prehistoric stone implements from the New Guinea Highlands. Oceania 34, 246–268 (1964).
Bulmer, S. Radiocarbon dates from New Guinea. J. Polynesian Soc. 73, 327–328 (1964).
Sutton, A., Mountain, M.-J., Aplin, K., Bulmer, S. & Denham, T. P. Archaeozoological records for the highlands of New Guinea: a review of current evidence. Aust. Archaeol. 69, 41–58 (2009).
Bulmer, R. The strategies of hunting in New Guinea. Oceania 38, 302–318 (1968).
Dwyer, P. D. An annotated list of mammals from Mt. Elimbari, Eastern Highlands Province, Papua New Guinea. Sci. New Guinea 10, 28–38 (1983).
Dwyer, P. D. The price of protein: five hundred hours of hunting in the New Guinea Highlands. Oceania 44, 278–293 (1974).
Flannery, T. F. Mammals of New Guinea (Cornell Univ. Press, 1995).
Leavesley, M. G. Trees to the Sky: Prehistoric Hunting in New Ireland, Papua New Guinea PhD thesis, Australian National Univ. (2004).
Sillitoe, P. Managing Mammals in New Guinea: Preying the Game in the Highlands (Routledge, 2003).
Clout, M. N. Determination of age in the brushtail possum using sections from decalcified molar teeth. New Zeal. J. Zool. 9, 405–408 (1982).
Lee-Thorp, J. A. et al. Isotopic evidence for an early shift to C4 resources by Pliocene hominins in Chad. Proc. Natl Acad. Sci. USA 109, 20369–20372 (2012).
Sponheimer, M. et al. Hominins, sedges, and termites: new carbon isotope data from the Sterkfontein valley and Kruger National Park. J. Hum. Evol. 48, 301–312 (2005).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
Acknowledgements
This project was funded by grants from the Natural Environmental Research Council and the Boise Fund, University of Oxford (to P.R.). We also thank the National Museum and Art Gallery of Papua New Guinea for supporting this research. J. Menzies provided helpful insight into the zoology. Finally, a special thanks goes to Sue Bulmer and her family for providing us with access to the materials and field notes for the site of Kiowa. We dedicate this paper to Sue, who sadly passed away during the writing of this paper—her legacy in Pacific archaeology and at the site of Kiowa remains.
Author information
Authors and Affiliations
Contributions
P.R., D.G., J.L.-T. and G.S. designed and performed the research, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Text; Supplementary Figures 1–3; Supplementary Tables 1–16; Supplementary References (PDF 738 kb)
Rights and permissions
About this article
Cite this article
Roberts, P., Gaffney, D., Lee-Thorp, J. et al. Persistent tropical foraging in the highlands of terminal Pleistocene/Holocene New Guinea. Nat Ecol Evol 1, 0044 (2017). https://doi.org/10.1038/s41559-016-0044
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-016-0044
This article is cited by
-
The deep human prehistory of global tropical forests and its relevance for modern conservation
Nature Plants (2017)
-
Palaeoecology: Agriculture emerges from the calm
Nature Ecology & Evolution (2017)