Abstract
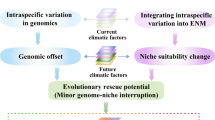
Climate adaptation and dispersal can determine a species’ response to climate change. However, quantifying how they can mitigate climate change risks remains a challenge. Here we combine ecological genomic, niche modelling and landscape genetic approaches to reveal similar population-level vulnerability for a keystone species and its two beneficiary species in an alpine grassland ecosystem in the Qinghai–Tibetan Plateau. We use climate-associated genotypes to identify population-level adaptation and model maladaptation with and without dispersal and find that contemporary populations in southwestern ranges are the most vulnerable to climate change. This vulnerability cannot be mitigated by dispersal to more suitable niches because of climate maladaptation and landscape barriers. Overall, combined multiple climate change risk estimates in coevolving species can be used to improve climate change vulnerability assessments beyond what can be learned from a single species or modelling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The resequencing data of 11 WR snowfinch and RN snowfinch individuals from ref. 22 can be found in Short Read Archive under the project number PRJNA417520 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA417520). The resequencing data of the plateau pika from ref. 21 can be found in the National Genomics Data Centre (https://db.cngb.org/) under the accession number CNP0003365 (https://db.cngb.org/search/project/CNP0003365/). Sequencing data generated in this study have been deposited in the National Genomics Data Centre (https://db.cngb.org/) under the accession number CNP0004029 (https://db.cngb.org/search/project/CNP0004029/).
Code availability
Datasets and analysis scripts can be found at GitHub: https://github.com/willright28/Tibet-mammals-and-birds (ref. 95).
References
Scheffers, B. R. et al. The broad footprint of climate change from genes to biomes to people. Science 354, aaf7671 (2016).
Urban, M. C. Accelerating extinction risk from climate change. Science 348, 571–573 (2015).
Smith, A. B. et al. Niche estimation above and below the species level. Trends Ecol. Evol. 34, 260–273 (2019).
Gotelli, J. N. & Stanton-Geddes, J. Climate change, genetic markers and species distribution modelling. J. Biogeogr. 42, 1577–1585 (2015).
Hällfors, M. H. et al. Addressing potential local adaptation in species distribution models: implications for conservation under climate change. Ecol. Appl. 26, 1154–1169 (2016).
Mendoza-Gonzalez, G. et al. Ecological niche modeling of coastal dune plants and future potential distribution in response to climate change and sea level rise. Glob. Change Biol. 19, 2524–2535 (2013).
Razgour, O. et al. Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl Acad. Sci. USA 116, 10418–10423 (2019).
Saunders, S. P. et al. Community science validates climate suitability projections from ecological niche modeling. Ecol. Appl. 30, e02128 (2020).
Ruegg, K. et al. Ecological genomics predicts climate vulnerability in an endangered southwestern songbird. Ecol. Lett. 21, 1085–1096 (2018).
Bay, R. A. et al. Genomic signals of selection predict climate driven population declines in a migratory bird. Science 359, 83–86 (2018).
Gougherty, A. V. et al. Maladaptation, migration and extirpation fuel climate change risk in a forest tree species. Nat. Clim. Change 11, 166–171 (2021).
Walters, R. J. & Berger, D. Implications of existing local (mal)adaptations for ecological forecasting under environmental change. Evol. Appl. 12, 1487–1502 (2019).
Laverdière, J. P. et al. Breeding for adaptation to climate change: genomic selection for drought response in a white spruce multi-site polycross test. Evol. Appl. 15, 383–402 (2021).
Vitt, P. et al. Assisted migration of plants: changes in latitudes, changes in attitudes. Biol. Conserv. 143, 18–27 (2010).
VanDerWal, J. et al. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Change 3, 239–243 (2013).
Fitzpatrick, M. C. & Keller, S. R. Ecological genomics meets community-level modelling of biodiversity: mapping the genomic landscape of current and future environmental adaptation. Ecol. Let. 18, 1–16 (2015).
Wingfield, J. C. et al. Organism-environment interactions in a changing world: a mechanistic approach. J. Ornithol. 152, S279–S288 (2011).
Favre, A. et al. The role of the uplift of the Qinghai–Tibetan Plateau for the evolution of Tibetan biotas. Biol. Rev. 90, 236–253 (2015).
Rahbek, C. et al. Building mountain biodiversity: geological and evolutionary processes. Science 365, 1114–1119 (2019).
Galbreath, K. E., Hafner, D. J. & Zamudio, K. R. When cold is better: climate-driven elevation shifts yield complex patterns of diversification and demography in an alpine specialist (American pika, Ochotona princeps). Evolution 63, 2848–2863 (2009).
Ge, D. Y. et al. Genomic consequences of and demographic response to pervasive hybridization over time in climate-sensitive pikas. Mol. Biol. Evol. 40, msac274 (2023).
She, H. et al. Quantifying adaptive divergence of the snowfinches in a common landscape. Divers. Distrib. 28, 2579–2592 (2022).
Lai, C. H. & Smith, A. T. Keystone status of plateau pikas (Ochotona curzoniae): effect of control on biodiversity of native birds. Biodivers. Conserv. 12, 1901–1912 (2003).
Sumbh, O. & Hof, A. R. Can pika hold the umbrella? Understanding the current and future umbrella potential of keystone species pika (Ochotona spp.). Glob. Ecol. Conserv. 38, e02247 (2022).
Renner, S. S. & Zohner, C. M. Climate change and phonological mismatch in trophic interactions among plants, insects and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165–182 (2018).
Weaver, S. A. & Mallinger, E. R. A specialist been and its host plants experience phonological shifts at different rates in response to climate change. Ecology 103, e3658 (2022).
Khaliq, I. et al. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B 281, 20141097 (2014).
Li, D. et al. Coping with extremes: convergences of habitat use, territoriality and diet in summer but divergences in winter between two sympatric snow finches on the Qinghai–Tibet Plateau. Integr. Zool. 15, 533–543 (2020).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Endelman, J. B. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome 4, 250 (2011).
Gienapp, P. et al. Genomic quantitative genetics to study evolution in the wild. Trends Ecol. Evol. 32, 897–908 (2020).
Lasky, J. R. et al. Genome–environment associations in sorghum landraces predict adaptive traits. Sci. Adv. 1, e1400218 (2015).
Wallace, J. M. et al. Global warming and winter weather. Science 343, 729–730 (2014).
Ummenhofer, C. C. & Meehl, G. A. Extreme weather and climate events with ecological relevance: a review. Philos. Trans. R. Soc. B 372, 13 (2017).
Fournier-Level, A. et al. A map of local adaptation in Arabidopsis thaliana. Science 34, 86–80 (2011).
Frichot, E. et al. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol. Biol. Evol. 30, 1687–1699 (2013).
Forester, B. R. et al. Detecting spatial genetic signatures of local adaptation in heterogeneous landscapes. Mol. Ecol. 25, 104–120 (2016).
O’neill, B. C. et al. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 9, 3461–3482 (2016).
Riahi, K. et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob. Environ. Change 42, 153–168 (2017).
Ellis, N. et al. Gradient forests: calculating importance gradients on physical predictors. Ecology 93, 156–168 (2012).
Ferrier, S. et al. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 13, 252–264 (2007).
Thuiller, W., Lafourcade, B., Engler, R. & Araújo, M. B. BIOMOD–a platform for ensemble forecasting of species distributions. Ecography 32, 369–373 (2009).
Petkova, D. et al. Visualizing spatial population structure with estimated effective migration surfaces. Nat. Genet. 48, 94–100 (2016).
Clarke, R. T. et al. Confidence limits for regression relationships between distance matrices: estimating gene flow with distance. J. Agric. Biol. Environ. Stat. 7, 361–372 (2002).
Dahlback, A., Gelsor, N., Stamnes, J. J. & Gjessing, Y. UV measurements in the 3,000–5,000 m altitude region in Tibet. J. Geophys. Res. Atmos. 112, 1984–2012 (2007).
Qu, Y. & Lei, F. Comparative phylogeography of two endemic birds of the Tibetan plateau, the white-rumped snow finch (Onychostruthus taczanowskii) and the Hume’s ground tit (Pseudopodoces humilis). Mol. Phylogenet. Evol. 51, 312–326 (2009).
Lei, F., Qu, Y. & Song, G. Species diversification and phylogeographical patterns of birds in response to the uplift of the Qinghai–Tibet plateau and Quaternary glaciations. Curr. Zool. 60, 149–161 (2014).
Lv, X. et al. Continental refugium in the Mongolian Plateau during Quanternary glacial oscillations: phylogeography and niche modeling of the endemic desert hamster, Phodopus roborovskii. PLoS ONE 11, e0148182 (2016).
Shi, Y. F. Characteristics of late Quaternary monsoonal glaciation on the Qinghai–Tibetan plateau and in East Asia. Quat. Int. 97–98, 79–91 (2002).
Zhang, D., Liu, F. & Bing, J. Eco-environmental effects of the Qinghai–Tibet plateau uplift during the Quaternary in China. Environ. Geol. 39, 1352–1358 (2000).
Clements, F. E. Plant Succession, an Analysis of the Development of Vegetation (Carnegie Institution, 1916).
Gleason, H. A. The individualistic concept of the plant association. Bull. Torrey Bot. Club 53, 7–26 (1926).
Pausas, J. G. & Bond, W. J. Alternative biome states challenge the modeling of species’ niche shifts under climate change. J. Ecol. 109, 3962–3971 (2021).
Johnson, S. A., Ober, H. & Adams, D. C. Are keystone species effective umbrellas for habitat conservation? A spatially explicit approach. J. Nat. Conserv. 37, 47–55 (2017).
Barrio, I. C. & Hik, D. S. Good neighbours? Determinants of aggregation and segregation among alpine herbivores. Ecoscience 20, 276–282 (2013).
Rhoné, B. et al. Pearl millet genomic vulnerability to climate change in West Africa highlights the need for regional collaboration. Nat. Commun. 11, 5274 (2020).
Foden, W. B. et al. Climate change vulnerability assessment of species. WIREs Clim. Change 10, e551 (2019).
Beck, J., Böller, M., Erhardt, A. & Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 19, 10–15 (2014).
Maldonado, C. et al. Estimating species diversity and distribution in the era of Big Data: to what extent can we trust public databases? Glob. Ecol. Biogeogr. 24, 973–984 (2015).
Van Beest, F. M. et al. Rapid shifts in Arctic tundra species’ distributions and inter-specific range overlap under future climate change. Divers. Distrib. 27, 1706–1718 (2021).
Scott, P. A. et al. Individual heterozygosity predicts translocation success in threated desert tortoises. Science 370, 1086–1089 (2020).
Zhang, Y. Integration dataset of Tibet Plateau boundary. A big earth data platform for three poles. National Tibetan Plateau Data Center https://doi.org/10.11888/Geogra.tpdc.270099 (2019).
Sjodin, B. M. F., Galbreath, K. E., Lanier, H. C. & Russello, M. A. Chromosome-level reference genome assembly for the American pika (Ochotona princeps). J. Hered. 112, 549–557 (2021).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Qu, Y. et al. The evolution of ancestral and species-specific adaptations in snowfinches at the Qinghai–Tibet Plateau. Proc. Natl Acad. Sci. USA 118, e2012398118 (2021).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
McInnes, L., Healy, J., Saul, N. & Großberger, L. Umap: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Speakman, J. R. et al. Surviving winter on the Qinghai–Tibetan plateau: pikas suppress energy demands and exploit yak feces to survive winter. Proc. Natl Acad. Sci. USA 118, e2100707118 (2021).
Song, S., Chen, J., Jiang, B. & Liu, N. Variation in egg and clutch size of the Black Redstart (Phoenicurus ochruros) at the northeastern edge of the Qinghai–Tibetan Plateau. Avian Res. 7, 20 (2016).
Rellstab, C. et al. A practical guide to environmental assocaition analysis in landscape genomics. Mol. Ecol. 24, 4348–4370 (2015).
Cui, T. et al. Evaluation of temperature and precipitation simulations in CMIP6 models over the Tibetan Plateau. Earth Space Sci. 8, e2020EA001620 (2021).
Keenan, K. et al. diveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol. Evol. 4, 782–788 (2013).
Denoël, M. & Ficetola, G. F. Using kernels and ecological niche modeling to delineate conservation areas in an endangered path-breeding phenotype. Ecol. Appl. 15, 1922–1931 (2015).
Ma, L. et al. Predicting range shifts of pikas (Mammalia, Ochotonidae) in China under scenarios incorporating land use change, climate change and dispersal limitations. Divers. Distrib. 27, 2384–2396 (2021).
Boria, R. A., Olson, L. E., Goodman, S. M. & Anderson, R. P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77 (2014).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017).
Wisz, M. S. et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 88, 15–30 (2013).
Han, L. et al. Preferred prey reduce species realized niche shift and improve range expansion prediction. Sci. Total Environ. 859, 160370 (2013).
Broennimann, O. et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21, 481–497 (2012).
Araújo, M. B. et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 5, eaat4858 (2019).
Vignali, S. et al. SDMtune: an R package to tune and evaluate species distribution models. Ecol. Evol. 10, 11488–11506 (2020).
Owens, H. L. et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 263, 10–18 (2013).
Akaike, H. New look at statistical-model identification. IEEE Trans. Autom. Control AC19, 716–723 (1974).
Phillips, S. J. et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197 (2009).
Shipley, B. R. et al. megaSDM: integrating dispersal and time-step analyses into species distribution models. Ecography 2022, e05450 (2022).
Bellard, C. et al. Will climate change promote future invasions? Glob. Change Biol. 19, 3740–3748 (2013).
Liu, C. R., White, M. & Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 40, 778–789 (2013).
Peterman, W. E. & Jarman, S. ResistanceGA: an R package for the optimization of resistance surfaces using genetic algorithms. Methods Ecol. Evol. 9, 1638–1647 (2018).
Bates, D. et al. Fitting linear mixed effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Anderson, D. R. & Burnham, K. P. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manage. 66, 912–918 (2002).
Van Strien, M. J., Keller, D. & Holderegger, R. A new analytical approach to landscape genetic modelling: least-cost transect analysis and linear mixed models. Mol. Ecol. 21, 4010–4023 (2012).
Bartoń, K. MuMIn: multi-model inference. R version 1.9.13. CRAN https://CRAN.R-project.org/package=MuMIn (2013).
Chen, Y. et al. Code and data fo ‘Alpine burrow-sharing mammals and birds show similar population-level climate change risks’. GitHub https://github.com/willright28/Tibet-mammals-and-birds (2023).
Acknowledgements
We sincerely thank H. Qiao and X. Liu for their facilitating ecological niche modelling. This research was funded by the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0501 to Y.Q., 2019QZKK0402 to D.G. and 2019QZKK0304-02 to F.L.), the National Natural Science Foundation of China (NSFC32020103005 to Y.Q., NSFC 32070434 to G.S. and NSFC 32170426 to D.G.), the Third Xinjiang Scientific Expedition and Research (2022xjkk0205 to Y.Q.) and the Swedish Research Council (621-2017-3693 to P.G.P.E.).
Author information
Authors and Affiliations
Contributions
Y.Q., D.G., P.G.P.E., F.L. and Q.Y. conceived, designed and refined the study; Y.C., D.G., P.G.P.E. and Y.Q. performed data analysis with assistance from G.S., Z.W. and X.L.; Y.Q., Y.C. and P.G.P.E. wrote the paper with edits from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Anouschka Hof, Devin R. de Zwaan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Population genetic structure of the three species.
Uniform manifold approximation and projection (a) and Admixture (b) do not detect clearly genetic divergence and the optimal number of clusters (k) explaining variation among populations is k = 1. Dark colored circles in (a) show individuals collected from north part of ranges, while light colored circles are those from south part of range.
Extended Data Fig. 2 The climatically adapted phenotypes (calculating as genome-estimated breeding values, GEBVs) are not uniformed distributed and vary with the gradients of the three climatic variables.
Solid lines and shadows show loess fitting curve and 95% confidence interval. These curves reflect that the GEBVs change across the maximum temperatures in the warmest month (a, bio5), the minimum temperature in the coldest month (b, bio6) and seasonal precipitation (c, bio15), suggesting different reactions to the given climatic variable. Large and small GEBVs values can be considered warm-wet adapted phenotypes (that is, sampling sites in southwestern parts) and cold-dry adapted phenotypes (that is, sampling sites in northeastern parts).
Extended Data Fig. 3 The heterozygosity of randomly extracted SNPs does not significantly correlate with the three climatic variables (grey dots and lines), while that of climate associated SNPs shows significant correlation with the three climatic variables.
Solid lines and shadows showed linear regression fitting curve and 95% confidence interval. (a) maximum temperatures in the warmest month (bio5), (b) the minimum temperature in the coldest month (bio6), (c) seasonal precipitation (bio15). These results indicate that the potential role of these candidate SNPs on local climate adaptation is not confounded by population genetic structure.
Extended Data Fig. 4 GradientForest predicted local genetic offsets (GO) of the three species in response to future climate changes.
(a)-(d) Local genetic offset modelling shows the high risk for the populations in southwestern parts and low risk for the populations in the northeastern parts of ranges. Left, plateau pika (n = 66); mid, WR-snowfinch (n = 68); right, RN-snowfinch (n = 55). (a) 2070 SSP1-2.6, (b) 2070 SSP5-8.5, (c) 2100 SSP1-2.6, (d) 2100 SSP5-8.5. (e) In all three species, local genetic offset revealed similar but magnitude-dependent spatial patterns under the different climate scenarios (n = 50,000 2.5-min grids). All modelling results consistently suggest that the local populations of the three species vary in their vulnerability to climate change, and that the populations in the southwestern parts of the ranges are most maladapted to future climate. The box plots show the median (center line) and 25th-75th percentiles (box limits). The whiskers extend to the top/bottom to the maxima and minima.
Extended Data Fig. 5 Generalized dissimilarity modelling (GDM) predicted local genetic offsets (GO) of the three species in response to future climate changes.
(a)-(d) Local genetic offset modelling shows the high climate risk for the populations in southwestern parts and low risk for the populations in the northeastern parts of ranges. Left, plateau pika (n = 66); mid, WR-snowfinch (n = 68); right, RN-snowfinch (n = 55). (a) 2070 SSP1-2.6, (b) 2070 SSP5-8.5, (c) 2100 SSP1-2.6, (d) 2100 SSP5-8.5. (e) In all three species, local genetic offset revealed similar but magnitude-dependent spatial patterns under the different climate scenarios (n = 50,000 2.5-min grids). All modelling results consistently suggest that the local populations of the three species vary in their vulnerability to climate change, and that the populations in the southwestern parts of the ranges are most maladapted to future climate. The box plots show the median (center line) and 25th-75th percentiles (box limits). The whiskers extend to the top/bottom to the maxima and minima.
Extended Data Fig. 6 Ecological niche modelling predicted suitable niches of the three species under current and future climate conditions. The ecological niche modelling took biointeraction into accounted.
(a)-(e) Projections of niches suitable for the three species. Left, plateau pika; mid, WR-snowfinch; right, RN-snowfinch. (a) Current time, (b) 2070 SSP1-2.6, (c) 2070 SSP5-8.5, (d) 2100 SSP1-2.6, (e) 2100 SSP5-8.5. Much of the areas in the southwestern parts of ranges will reduce or lose the suitable niches, but those in the northeastern parts will remain climatically suitable for all three species.
Extended Data Fig. 7 GradientForest predicted forward genetic offsets (FGO) of the three species in response to future climate changes.
(a)-(d) Forward genetic offset modelling shows the high risk for the populations in southwestern parts and low risk for the populations in the northeastern parts of ranges. Left, plateau pika (n = 66); mid, WR-snowfinch (n = 68); right, RN-snowfinch (n = 55). (a) 2070 SSP1-2.6, (b) 2070 SSP5-8.5, (c) 2100 SSP1-2.6, (d) 2100 SSP5-8.5. These results show the lowest forward genetic offsets are found for the populations in the northeastern parts of the ranges and the highest genetic offsets for the populations in the southwestern parts of the ranges.
Extended Data Fig. 8 GradientForest predicted reverse genetic offsets (RGO) of the three species in response to future climate changes.
(a)-(d) Reverse genetic offset modelling shows the high risk for the populations in southwestern parts and low risk for the populations in the northeastern parts of ranges. Left, plateau pika (n = 66); mid, WR-snowfinch (n = 68); right, RN-snowfinch (n = 55). (a) 2070 SSP1-2.6, (b) 2070 SSP5-8.5, (c) 2100 SSP1-2.6, (d) 2100 SSP5-8.5. These results show the lowest reverse genetic offsets are found for the populations in the northeastern parts of the ranges and the highest genetic offsets for the populations in the southwestern parts of the ranges.
Extended Data Fig. 9 Landscape genetic analysis predicted the density of dispersals between populations based on the effect of topology for plateau pika, land cover for WR-snowfinch and climatic conditions for RN-snowfinch.
Landscape barriers limit dispersal between southwestern and northeastern populations, and the habitat barriers would be further exacerbated for the RN-snowfinch but remain the same for plateau pika and WR-snowfinch under future climate. Left, plateau pika; mid, WR-snowfinch; right, RN-snowfinch. From top to bottom, current, 2070 SSP1-2.6, 2070 SSP5-8.5, 2100 SSP1-2.6, 2100 SSP5-8.5.
Extended Data Fig. 10 Projection of niche suitability using ecological niche modelling without considering biointeraction.
Three species show high similarity in their current and future suitable niches (left), supporting by high Pearson’s correlation coefficients (middle, rs = 0.81-0.96, P < 2.66e-22, two-tailed Pearson’s correlation) and Schoener’s D niche similarity scores (right, Schoener’s Ds = 0.81-0.93). These results suggest that the three species show similar population-level climate change vulnerability.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–5.
Supplementary Data 1
Sampling information of the three species used in this study. We sampled and sequenced 66, 68 and 55 individual plateau pikas, WR snowfinches and RN snowfinches across their distribution ranges.
Supplementary Data 2
Statistics of the sequencing data of the three species used in this study. We generated the high-density genomic datasets (mean sequencing depth of 18×–21×) comprising 189 individuals representing the three species across their distribution ranges.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Ge, D., Ericson, P.G.P. et al. Alpine burrow-sharing mammals and birds show similar population-level climate change risks. Nat. Clim. Chang. 13, 990–996 (2023). https://doi.org/10.1038/s41558-023-01772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-023-01772-8
This article is cited by
-
Alpine burrow-sharing mammals and birds show similar population-level climate change risks
Nature Climate Change (2023)