Abstract
Climate change can drive shifts in the seasonality of marine productivity, with consequences for the marine food web. However, these alterations in phytoplankton bloom phenology (initiation and peak timing), and the underlying drivers, are not well understood. Here, using a 30-member Large Ensemble of climate change projections, we show earlier bloom initiation in most ocean regions, yet changes in bloom peak timing vary widely by region. Shifts in both initiation and peak timing are induced by a subtle decoupling between altered phytoplankton growth and zooplankton predation, with increased zooplankton predation (top-down control) playing an important role in altered bloom peak timing over much of the global ocean. Only in limited regions is light limitation a primary control for bloom initiation changes. In the extratropics, climate-change-induced phenological shifts will exceed background natural variability by the end of the twenty-first century, which may impact energy flow in the marine food webs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The 30-member GFDL-ESM2M ensemble simulations53 used in this study are available through the data transfer service Globus (https://www.globus.org). In the server (http://poseidon.princeton.edu), daily surface ocean variables are available under: /GFDL_ESM2M/ENSEMBLE_RCP85/OCN/OCN_1D_1×1/ and monthly mean ocean fields are under /GFDL_ESM2M/ENSEMBLE_RCP85/OCN/OCN_1M_1×1. The MODIS-Aqua Level-3 Binned Chlorophyll Data50 are available at https://oceancolor.gsfc.nasa.gov.
Code availability
The codes used to analyse data and generate all figures are based on Python with associated standard Python packages (Xarray, NumPy, SciPy, Matplotlib, Cartopy and so on). The codes54 are available from https://doi.org/10.5281/zenodo.6301884.
References
Bindoff, N. L. et al. in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (eds Pörtner, H.-O. et al.) 447–587 (Cambridge Univ. Press,2019).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Fu, W., Randerson, J. T. & Moore, J. K. Climate Change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models. Biogeosciences 13, 5151–5170 (2016).
Kwiatkowski, L. et al. Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17, 3439–3470 (2020).
Cushing, D. H. in Advances in Marine Biology Vol. 26 (eds Blaxter, J. H. S. & Southward, A. J.) 249–293 (Academic Press, 1990).
Durant, J., Hjermann, D., Ottersen, G. & Stenseth, N. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283 (2007).
Edwards, M. & Richardson, A. J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (2004).
Durant, J. M. et al. Contrasting effects of rising temperatures on trophic interactions in marine ecosystems. Sci. Rep. 9, 15213 (2019).
Penuelas, J., Rutishauser, T. & Filella, I. Phenology feedbacks on climate change. Science 324, 887–888 (2009).
Ruosteenoja, K., Markkanen, T. & Räisänen, J. Thermal seasons in northern Europe in projected future climate. Int. J. Climatol. 40, 4444–4462 (2020).
Jeong, S.-J., Ho, C.-H., Gim, H.-J. & Brown, M. E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 17, 2385–2399 (2011).
Zhou, B., Zhai, P., Chen, Y. & Yu, R. Projected changes of thermal growing season over Northern Eurasia in a 1.5 °C and 2 °C warming world. Environ. Res. Lett. 13, 035004 (2018).
Chen, M., Melaas, E. K., Gray, J. M., Friedl, M. A. & Richardson, A. D. A new seasonal-deciduous spring phenology submodel in the Community Land Model 4.5: impacts on carbon and water cycling under future climate scenarios. Glob. Change Biol. 22, 3675–3688 (2016).
Kahru, M., Brotas, V., Manzano-Sarabia, M. & Mitchell, B. G. Are phytoplankton blooms occurring earlier in the Arctic?. Glob. Change Biol. 17, 1733–1739 (2011).
Marchese, C. et al. Changes in phytoplankton bloom phenology over the North Water (NOW) polynya: a response to changing environmental conditions. Polar Biol. 40, 1721–1737 (2017).
Friedland, K. D. et al. Phenology and time series trends of the dominant seasonal phytoplankton bloom across global scales. Glob. Ecol. Biogeogr. 27, 551–569 (2018).
Winder, M. & Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 698, 5–16 (2012).
Asch, R. G., Stock, C. A. & Sarmiento, J. L. Climate change impacts on mismatches between phytoplankton blooms and fish spawning phenology. Glob. Change Biol. 25, 2544–2559 (2019).
Henson, S., Cole, H., Beaulieu, C. & Yool, A. The impact of global warming on seasonality of ocean primary production. Biogeosciences 10, 4357–4369 (2013).
Henson, S. A., Cole, H. S., Hopkins, J., Martin, A. P. & Yool, A. Detection of climate change-driven trends in phytoplankton phenology. Glob. Change Biol. 24, e101–e111 (2018).
Hashioka, T., Sakamoto, T. T. & Yamanaka, Y. Potential impact of global warming on North Pacific spring blooms projected by an eddy-permitting 3-D ocean ecosystem model. Geophys. Res. Lett. 36, L20604 (2009).
Behrenfeld, M. J. & Boss, E. S. Resurrecting the ecological underpinnings of ocean plankton blooms. Annu. Rev. Mar. Sci. 6, 167–194 (2014).
Behrenfeld, M. J. Climate-mediated dance of the plankton. Nat. Clim. Change 4, 880–887 (2014).
Schlunegger, S. et al. Time of Emergence and Large Ensemble intercomparison for ocean biogeochemical trends. Glob. Biogeochem. Cycles 34, e2019GB006453 (2020).
Sverdrup, H. U. On conditions for the vernal blooming of phytoplankton. ICES J. Mar. Sci. 18, 287–295 (1953).
Sommer, U. & Lewandowska, A. Climate change and the phytoplankton spring bloom: warming and overwintering zooplankton have similar effects on phytoplankton. Glob. Change Biol. 17, 154–162 (2011).
Behrenfeld, M. J. Abandoning Sverdrup’s critical depth hypothesis on phytoplankton blooms. Ecology 91, 977–989 (2010).
Behrenfeld, M. J., Doney, S. C., Lima, I., Boss, E. S. & Siegel, D. A. Annual cycles of ecological disturbance and recovery underlying the subarctic Atlantic spring plankton bloom. Glob. Biogeochem. Cycles 27, 526–540 (2013).
Arteaga, L. A., Boss, E., Behrenfeld, M. J., Westberry, T. K. & Sarmiento, J. L. Seasonal modulation of phytoplankton biomass in the Southern Ocean. Nat. Commun. 11, 5364 (2020).
Behrenfeld, M. J. & Boss, E. S. Student’s tutorial on bloom hypotheses in the context of phytoplankton annual cycles. Glob. Change Biol. 24, 55–77 (2018).
Schlunegger, S. et al. Emergence of anthropogenic signals in the ocean carbon cycle. Nat. Clim. Change 9, 719–725 (2019).
Dunne, J. P. et al. GFDL’s ESM2 Global Coupled Climate–Carbon Earth System Models. Part II: Carbon system formulation and baseline simulation characteristics. J. Clim. 26, 2247–2267 (2013).
Dunne, J. P. et al. GFDL’s ESM2 Global Coupled Climate–Carbon Earth System Models. Part I: Physical formulation and baseline simulation characteristics. J. Clim. 25, 6646–6665 (2012).
Rodgers, K. B., Lin, J. & Frölicher, T. L. Emergence of multiple ocean ecosystem drivers in a large ensemble suite with an Earth system model. Biogeosciences 12, 3301–3320 (2015).
Cabré, A., Shields, D., Marinov, I. & Kostadinov, T. S. Phenology of size-partitioned phytoplankton carbon-biomass from ocean color remote sensing and CMIP5 models. Front. Mar. Sci. 3, 39 (2016).
Ji, R., Edwards, M., Mackas, D. L., Runge, J. A. & Thomas, A. C. Marine plankton phenology and life history in a changing climate: current research and future directions. J. Plankton Res. 32, 1355–1368 (2010).
Henson, S. A., Beaulieu, C. & Lampitt, R. Observing climate change trends in ocean biogeochemistry: when and where. Glob. Change Biol. 22, 1561–1571 (2016).
Hegerl, G. C. et al. Detecting greenhouse-gas-induced climate change with an optimal fingerprint method. J. Clim. 9, 2281–2306 (1996).
Massonnet, F. et al. Constraining projections of summer Arctic sea ice. Cryosphere 6, 1383–1394 (2012).
Chemke, R., Zanna, L. & Polvani, L. M. Identifying a human signal in the North Atlantic warming hole. Nat. Commun. 11, 1540 (2020).
Behrenfeld, M. J. et al. Revaluating ocean warming impacts on global phytoplankton. Nat. Clim. Change 6, 323–330 (2016).
Geider, R., MacIntyre, H. & Kana, T. Dynamic model of phytoplankton growth and acclimation: responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 148, 187–200 (1997).
Wassmann, P. & Reigstad, M. Future Arctic Ocean seasonal ice zones and implications for pelagic–benthic coupling. Oceanography 24, 220–231 (2011).
Laufkötter, C. et al. Drivers and uncertainties of future global marine primary production in marine ecosystem models. Biogeosciences 12, 6955–6984 (2015).
Nakamura, Y. & Oka, A. CMIP5 model analysis of future changes in ocean net primary production focusing on differences among individual oceans and models. J. Oceanogr. 75, 441–462 (2019).
Marinov, I., Doney, S. C. & Lima, I. D. Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences 7, 3941–3959 (2010).
Henson, S. A., Cael, B. B., Allen, S. R. & Dutkiewicz, S. Future phytoplankton diversity in a changing climate. Nat. Commun. 12, 5372 (2021).
Elson, P. et al. SciTools/cartopy: v0.20.2. Zenodo https://doi.org/10.5281/zenodo.1182735 (2022).
Frölicher, T. L. et al. Dominance of the Southern Ocean in anthropogenic carbon and heat uptake in CMIP5 models. J. Clim. 28, 862–886 (2015).
MODIS-Aqua Level 3 Binned Chlorophyll Data Version R2018.0 (NASA Ocean Biology Processing Group, 2017); https://doi.org/10.5067/AQUA/MODIS/L3B/CHL/2018
Fay, A. R. & McKinley, G. A. Global open-ocean biomes: mean and temporal variability. Earth Syst. Sci. Data 6, 273–284 (2014).
Behrenfeld, M. J., Doney, S. C., Lima, I., Boss, E. S. & Siegel, D. A. Reply to a comment by Stephen M. Chiswell on: ‘Annual cycles of ecological disturbance and recovery underlying the subarctic Atlantic spring plankton bloom’ by M. J. Behrenfeld et al. (2013). Global Biogeochem. Cycles 27, 1294–1296 (2013).
Geophysical Fluid Dynamics Laboratory Earth System Model 2 (GFDL-ESM2M) 30-Member Ensemble Simulations, Historical (1950–2005) and RCP8.5 (2006–2010) Scenario (NOAA GFDL, 2019); http://poseidon.princeton.edu
Yamaguchi, R. Notebooks for ‘Trophic level decoupling drives future change in phytoplankton bloom phenology’ by Yamaguchi et al. (2022). Zenodo https://doi.org/10.5281/zenodo.6301884 (2022).
Acknowledgements
R.Y., K.B.R., K.S. and A.T. were supported by the Institute for Basic Science (IBS), Republic of Korea, under IBS-R028-D1. S.S. acknowledges support from the National Science Foundation’s Southern Ocean Carbon and Climate Observations and Modeling (SOCCOM) Project under the NSF Award PLR-1425989, with additional support from the National Oceanic and Atmospheric Administration (NOAA) and NASA Award NNX17AI75G. D.B. acknowledges support from NOAA under Ecosystem and Harmful Algal Bloom (ECOHAB) Award NA18NOS4780174. R.D.S. acknowledges support from NOAA (NA18OAR4320123). We thank H.-G. Lim for constructive suggestions on the manuscript. ESM2M output analysis was conducted on the IBS/ICCP supercomputer ‘Aleph’, 1.43 peta flops high-performance Cray XC50-LC Skylake computing system with 18,720 processor cores, 9.59 PB storage and 43 PB tape archive space. High Performance Computing resources for the ESM2M Large Ensemble simulations themselves were provided by NOAA Oceanic and Atmospheric Research/Geophysical Fluid Dynamics Laboratory.
Author information
Authors and Affiliations
Contributions
R.Y. and K.B.R. conceptualized the scientific framing of this study. R.Y. conducted all the analyses and wrote the initial draft of the manuscript. R.Y., K.B.R., A.T., K.S., S.S., D.B. and J.P.D. helped develop the scientific ideas and analytical methods, interpret the results and write the final draft of the manuscript. The ESM2M simulations were performed by S.S. and R.D.S., and postprocessing was provided by K.B.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Lionel Arteaga, Emmanuel Boss, Stephanie Henson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
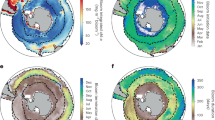
Extended Data Fig. 1 Biome map and biome-aggregated time series of observed and projected bloom initiation and peak timing.
In order from the pole, each basin (North (N) and South (S) Pacific (P), Atlantic (A), and Indian Ocean(I)) except for the North Indian Ocean has ice (ICE), subpolar seasonally stratified (SPSS), subtropical seasonally stratified (STSS), subtropical permanently stratified (STPS), and equatorial (EQU) biomes. Observational time series are computed from satellite-derived chlorophyll product (MODIS-Aqua) and the projections are simulated by the Geophysical Fluid Dynamics Laboratory Earth System Model 2 (GFDL-ESM2M) under Historical/RCP8.5 scenario. All time series are anomalies relative to satellite observational period means (2003-2019). The grey lines mark differences between bloom peak timing anomalies and initiation anomalies, indicating changes in the net growth period length. The shadings around the green and orange lines represent the two standard deviation range across the 30 ensemble members of ESM2M. Time of Emergence for the biome-aggregated changes in bloom initiation and peak timing is shown in green and orange vertical lines, respectively. If there is no corresponding vertical line in the panel, the phenological change will not emerge by the end of the simulation period. Note that the model time series are created without the resampling process (see Methods) in order to be consistent along the time-dimension of the model output from year 1990 to the end of the simulation.
Extended Data Fig. 2 Projected future changes in the phytoplankton bloom magnitude.
a, Ensemble mean climatology (1990–2020) of the phytoplankton bloom magnitude. b, Ensemble mean trends (1990–2100 under Historical/RCP8.5 scenario) of the bloom magnitude, simulated by the Geophysical Fluid Dynamics Laboratory Earth System Model 2 (GFDL-ESM2M). Regions where the trend is statistically insignificant (the 99% confidence level based on a t-test) are stippled. Black contours superimposed on the maps denote the biome boundaries used in this study. c, Biome-averaged changes (2080–2100 minus 1990–2010) in the bloom magnitude. In each ocean basin, ice (ICE), subpolar seasonally stratified (SPSS), subtropical seasonally stratified (STSS), subtropical permanently stratified (STPS), and equatorial (EQU) biomes are assigned in order from the pole (spatial map in Extend Data Fig. 1). Ensemble mean changes are shown as bars, and dots on each bar represent individual members’ changes. The stars at the end of bars indicate that the ensemble mean changes will emerge, that is, the climate-forced changes exceed the background internal variability, by the end of the 21st century. Note that, in panel c, another y-axis is provided on the right only for scaling the Northern Hemisphere ICE result.
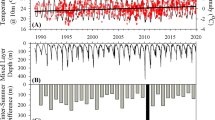
Extended Data Fig. 3 Mechanistic attribution of the bloom phenological changes for the subarctic North Pacific (150°W, 50°N).
a, Future (2080–2100) and present-day (1990–2010) annual cycles of surface chlorophyll concentration (Chl, g kg-1), b, chlorophyll accumulation rate (r ≡ dlnChl/dt, day-1) and its present-day term balance. r is determined by phytoplankton growth (μ) and loss rate (l), and temporal changes in Chl to Carbon ratio (θ) and mixed layer depth (h) (equation 1). c, Budget for the accumulation rate changes (Δr, future r minus present-day r). The bars with respect to the left axis are accumulation rate change and three changes that drives the Δr (equation 6). Changes in the growth and loss rates are also shown separately as lines with shading (right axis). d, Decomposition of the growth rate changes into changes in environmental drivers. The phytoplankton growth rate change is decomposed into changes in Temperature-, Nutrient-, Light-limitation (equation 8). e, Decomposition of the loss rate changes into changes in environmental drivers. The phytoplankton loss rate change is decomposed into changes in Temperature limitation and phytoplankton abundance (equation 9). The periods commonly shaded in light grey in a-d represent the periods between the present-day and future bloom initiation/peak timing determined from the annual cycle of r (b). All line shadings are the range of two standard deviations across the 30 ensemble members.
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2, Figs. 1–9 and Table 1.
Rights and permissions
About this article
Cite this article
Yamaguchi, R., Rodgers, K.B., Timmermann, A. et al. Trophic level decoupling drives future changes in phytoplankton bloom phenology. Nat. Clim. Chang. 12, 469–476 (2022). https://doi.org/10.1038/s41558-022-01353-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-022-01353-1
This article is cited by
-
Widespread changes in Southern Ocean phytoplankton blooms linked to climate drivers
Nature Climate Change (2023)