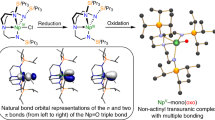
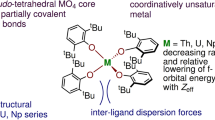
Abstract
Neptunium is an actinide element sourced from anthropogenic production, and, unlike naturally abundant uranium, its coordination chemistry is not well developed in all accessible oxidation states. High-valent neptunium generally requires stabilization from at least one metal–ligand multiple bond, and departing from this structural motif poses a considerable challenge. Here we report a tetrahedral molecular neptunium(V) complex ([Np5+(NPC)4][B(ArF5)4], 1-Np) (NPC = [NPtBu(pyrr)2]−; tBu = C(CH3)3; pyrr = pyrrolidinyl (N(C2H4)2); B(ArF5)4 = tetrakis(2,3,4,5,6-pentafluourophenyl)borate). Single-crystal X-ray diffraction, solution-state spectroscopy and density functional theory studies of 1-Np and the product of its proton-coupled electron transfer (PCET) reaction, 2-Np, demonstrate the unique bonding that stabilizes this reactive ion and establishes the thermochemical and kinetic parameters of PCET in a condensed-phase transuranic complex. The isolation of this four-coordinate, neptunium(V) complex reveals a fundamental reaction pathway in transuranic chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
X-ray data are available free of charge from the Cambridge Crystallographic Data Centre under reference ID nos. 2280905 (1-Np), 2280906 (2-Np) and 2280907 (Np-Cl). Additional spectroscopic and computational data are included in the supplementary data files. The source datasets generated and/or analysed during the current study, including computational coordinates/energies and processed spectroscopic data, have been deposited in the figshare database under accession code https://doi.org/10.6084/m9.figshare.24309463 (ref. 51). Source data are provided with this paper.
References
Dutkiewicz, M. S. et al. A terminal neptunium(V)–mono(oxo) complex. Nat. Chem. 14, 342–349 (2022).
Brown, J. L. et al. A linear trans-bis(imido) neptunium(V) actinyl analog: NpV(NDipp)2(tBu2bipy)2Cl (Dipp = 2,6-iPr2C6H3). J. Am. Chem. Soc. 137, 9583–9586 (2015).
Eller, P. G., Malm, J. G., Swanson, B. I. & Morss, L. R. Reactions of hexafluorides of uranium, neptunium and plutonium with nitrogen oxides and oxyfluorides. Synthesis and characterization of (NO)[NpF6] and (NO)[PuF6]. J. Alloys Comp. 269, 50–56 (1998).
Dutkiewicz, M. S. et al. Organometallic neptunium(III) complexes. Nat. Chem. 8, 797–802 (2016).
Asprey, L. B., Keenan, T. K., Penneman, R. A. & Sturgeon, G. D. Alkali fluoride complexes of pentavalent neptunium. Inorg. Nucl. Chem. Lett. 2, 19–21 (1966).
Asprey, L. B. & Penneman, R. A. Fluorine oxidation of tetravalent uranium and neptunium to the pentavalent state. J. Am. Chem. Soc. 89, 172 (1967).
Malm, J. G., Williams, C. W., Soderholm, L. & Morss, L. R. Preparation, chemical reactions and some physical properties of neptunium pentafluoride. J. Alloys Comp. 194, 133–137 (1993).
Samulski, E. T. & Karraker, D. G. Some ethoxide compounds of neptunium. J. Inorg. Nucl. Chem. 29, 993–996 (1967).
Rice, N. T. et al. Homoleptic imidophosphorane stabilization of tetravalent cerium. Inorg. Chem. 58, 5289–5304 (2019).
Rice, N. T. et al. Design, isolation and spectroscopic analysis of a tetravalent terbium complex. J. Am. Chem. Soc. 141, 13222–13233 (2019).
Rice, N. T. et al. Comparison of tetravalent cerium and terbium ions in a conserved, homoleptic imidophosphorane ligand field. Chem. Sci. 11, 6149–6159 (2020).
Rice, N. T. et al. Spectroscopic and electrochemical characterization of a Pr4+ imidophosphorane complex and the redox chemistry of Nd3+ and Dy3+ complexes. Dalton Trans. 51, 6696–6706 (2022).
Otte, K. S. et al. Divergent stabilities of tetravalent cerium, uranium and neptunium imidophosphorane complexes. Angew. Chem. Int. Ed. 62, e202306580 (2023).
Niklas, J. E., Studvick, C. M., Bacsa, J., Popov, I. A. & La Pierre, H. S. Ligand control of oxidation and crystallographic disorder in the isolation of hexavalent uranium mono-oxo complexes. Inorg. Chem. 62, 2304–2316 (2023).
Connelly, N. G. & Geiger, W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Stein, B. W., Kozimor, S. A. & Mocko, V. in Plutonium Handbook: Nuclear Science and Materials Science (ed. Clark, D. L.) Ch. 42 (American Nuclear Society, 2019).
Foster, J. P. & Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 102, 7211–7218 (1980).
Weinhold, F. & Landis, C. R. Valency and Bonding (Cambridge Univ. Press, 2003).
Zubarev, D. Y. & Boldyrev, A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 10, 5207–5217 (2008).
Bader, R. F. W. Atoms in molecules. Acc. Chem. Res. 18, 9–15 (1985).
Cremer, D. & Kraka, E. Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond? Angew. Chem. Int. Ed. 23, 627–628 (1984).
O’Grady, E. & Kaltsoyannis, N. On the inverse trans influence. Density functional studies of [MOX5]n− (M = Pa, n = 2; M = U, n = 1; M = Np, n = 0; X = F, Cl or Br). Dalton Trans. 2002, 1233–1239 (2002).
Denning, R. G. Electronic structure and bonding in actinyl ions and their analogs. J. Phys. Chem. A 111, 4125–4143 (2007).
Minasian, S. G. et al. Determining relative f and d orbital contributions to M-Cl covalency in MCl62− (M = Ti, Zr, Hf, U) and UOCl5− using Cl K-edge X-ray absorption spectroscopy and time-dependent density functional theory. J. Am. Chem. Soc. 134, 5586–5597 (2012).
Kosog, B., La Pierre, H. S., Heinemann, F. W., Liddle, S. T. & Meyer, K. Synthesis of uranium(VI) terminal oxo complexes: molecular geometry driven by the inverse trans-influence. J. Am. Chem. Soc. 134, 5284–5289 (2012).
La Pierre, H. S. & Meyer, K. Uranium-ligand multiple bonding in uranyl analogues, [L=U=L]n+ and the inverse trans influence. Inorg. Chem. 52, 529–539 (2013).
Lewis, A. J., Mullane, K. C., Nakamaru-Ogiso, E., Carroll, P. J. & Schelter, E. J. The inverse trans influence in a family of pentavalent uranium complexes. Inorg. Chem. 53, 6944–6953 (2014).
Köhler, L. et al. Insights into the electronic structure of a U(IV) amido and U(V) imido complex. Chem. Eur. J. 28, e202200119 (2022).
Wise, C. F., Agarwal, R. G. & Mayer, J. M. Determining proton-coupled standard potentials and X–H bond dissociation free energies in nonaqueous solvents using open-circuit potential measurements. J. Am. Chem. Soc. 142, 10681–10691 (2020).
Bruch, Q. J. et al. Dinitrogen reduction to ammonium at rhenium utilizing light and proton-coupled electron transfer. J. Am. Chem. Soc. 141, 20198–20208 (2019).
Lee, H. B., Britt, R. D. & Rittle, J. N–H bond dissociation free energy of a terminal iron phosphinimine. J. Coord. Chem. 75, 1804–1814 (2022).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Agarwal, R. G. et al. Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 122, 1–49 (2022).
Renshaw, J. C. et al. Bioreduction of uranium: environmental implications of a pentavalent intermediate. Environ. Sci. Technol. 39, 5657–5660 (2005).
Cologgi, D. L., Lampa-Pastirk, S., Speers, A. M., Kelly, S. D. & Reguera, G. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc. Natl Acad. Sci. USA 108, 15248–15252 (2011).
Lovley, D. R., Phillips, E. J. P., Gorby, Y. A. & Landa, E. R. Microbial reduction of uranium. Nature 350, 413–416 (1991).
Hou, X., McLachlan, J. R. & Dares, C. J. Electrochemical behaviour of uranium at a tripolyphosphate modified ITO electrode. Chem. Commun. 57, 10891–10894 (2021).
Dares, C. J., Lapides, A. M., Mincher, B. J. & Meyer, T. J. Electrochemical oxidation of 243Am(III) in nitric acid by a terpyridyl-derivatized electrode. Science 350, 652–655 (2015).
Hennig, C., Ikeda-Ohno, A., Tsushima, S. & Scheinost, A. C. The sulfate coordination of Np(IV), Np(V) and Np(VI) in aqueous solution. Inorg. Chem. 48, 5350–5360 (2009).
Bender, W. M. & Becker, U. Resolving the kinetics of individual aqueous reaction steps of actinyl (AnO2+ and AnO22+; An = U, Np and Pu) tricarbonate complexes with ferrous iron and hydrogen sulfide from first principles. Radiochim. Acta 108, 165–184 (2020).
Faizova, R., Fadaei-Tirani, F., Chauvin, A.-S. & Mazzanti, M. Synthesis and characterization of water stable uranyl(V) complexes. Angew. Chem. Int. Ed. 60, 8227–8235 (2021).
Faizova, R., Fadaei-Tirani, F., Bernier-Latmani, R. & Mazzanti, M. Ligand-supported facile conversion of uranyl(VI) into uranium(IV) in organic and aqueous media. Angew. Chem. 132, 6822–6825 (2020).
Bots, P. et al. Neptunium(V) and uranium(VI) reactions at the magnetite (111). Surface Geosci. 9, 81 (2019).
Collins, R. N. & Rosso, K. M. Mechanisms and rates of U(VI) reduction by Fe(II) in homogeneous aqueous solution and the role of U(V) disproportionation. J. Phys. Chem. A 121, 6603–6613 (2017).
Wander, M. C. F., Kerisit, S., Rosso, K. M. & Schoonen, M. A. A. Kinetics of triscarbonato uranyl reduction by aqueous ferrous iron: a theoretical study. J. Phys. Chem. 110, 9691–9701 (2006).
Chatelain, L. et al. Terminal uranium(V)-nitride hydrogenations involving direct addition or frustrated Lewis pair mechanisms. Nat. Commun. 11, 337 (2020).
Keener, M., Scopelliti, R. & Mazzanti, M. Nitride protonation and NH3 binding versus N-H bond cleavage in uranium nitrides. Chem. Sci. 12, 12610–12618 (2021).
Perales, D. et al. Conversion of uranium(III) anilido complexes to uranium(IV) imido complexes via hydrogen atom transfer. Organometallics 41, 606–616 (2022).
Nocera, D. G. Proton-coupled electron transfer: the engine of energy conversion and storage. J. Am. Chem. Soc. 144, 1069–1081 (2022).
La Pierre, H. S. et al. Source data for calculations, UV-vis-NIR, and NMR for a tetrahedral neptunium(V) complex. figshare https://doi.org/10.6084/m9.figshare.24309463 (2024).
Acknowledgements
We thank C. Windorff (NMSU) for his support during laboratory set-up and the initial transuranic synthesis. This material is based on work supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry programme under award no. DE-SC0019385 (J.E.N., K.S.O. and H.S.L.P.) at the Georgia Institute of Technology. Computational work was conducted using the computational resources at the Ohio Supercomputer Center and the ARCC HPC cluster at the University of Akron (C.M.S. and I.A.P.), as well as at the University of South Dakota, supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry programme under award no. DE-SC0023022 (S.R.C. and B.V.) and by the high-performance computing systems at the University of South Dakota, funded by the National Science Foundation under award no. OAC-1626516 (S.R.C. and B.V.).
Author information
Authors and Affiliations
Contributions
J.E.N., K.S.O. and H.S.L.P. conceived the idea presented in this publication. H.S.L.P., I.A.P. and B.V. supervised the project and acquired funding. J.E.N., K.S.O. and H.S.L.P. developed the syntheses and performed the spectroscopic characterization. Crystallographic characterization was performed by K.S.O., J.E.N., H.S.L.P., J.B. and F.K. Theoretical calculations and analysis were performed by C.M.S., S.R.C., B.V. and I.A.P. Analysis and visualization were performed by J.E.N., K.S.O., C.M.S., S.R.C., I.A.P., B.V. and H.S.L.P. The first draft was written by J.E.N., K.S.O., C.M.S. and S.R.C., with contributions from all authors. The paper was reviewed and edited by H.S.L.P., I.A.P. and B.V., with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Valerie Vallet and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PCET reactivity on the NMR timescale.
31P{1H} NMR spectra of 1-Np in THF-d8, prepared at t0 showing loss of 1-Np and ingrowth of 2-Np signals. Integration values are normalized to a sum of 1.0 and represent averages over independently processed and integrated data.
Extended Data Fig. 2 BDFE calculations.
Thermodynamic equations employed for calculating the N–H bond dissociation free energy of 2-Np through H atom transfer mediated by TEMPO-H.
Supplementary information
Supplementary Information
Supplementary Information (Experimental Details, Spectroscopic Data, Computational Details, References), Figs. 1–29 and Tables 1–13.
Supplementary Data 1
Crystallographic data for compound 1-Np; CCDC reference no. 2280905.
Supplementary Data 2
Crystallographic data for compound 2-Np; CCDC reference no. 2280906.
Supplementary Data 3
Crystallographic data for compound Np-Cl; CCDC reference no. 2280907.
Supplementary Data 4
DFT atomic coordinates.
Source data
Source Data Fig. 2
UV-vis-NIR source data for 1-Np at 8 mM and 0.1 mM.
Source Data Fig. 3
DFT single point energy calculations using an implicit solvent model of THF.
Source Data Fig. 4
31P NMR integration values at each time point and SD calculation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niklas, J.E., Otte, K.S., Studvick, C.M. et al. A tetrahedral neptunium(V) complex. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01529-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01529-6