Abstract
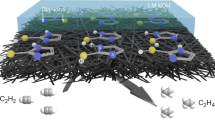
Hydrogen evolution is an important fuel-generating reaction that has been subject to mechanistic debate about the roles of monometallic and bimetallic pathways. The molecular iridium catalysts in this study undergo photoelectrochemical dihydrogen (H2) evolution via a bimolecular mechanism, providing an opportunity to understand the factors that promote bimetallic H–H coupling. Covalently tethered diiridium catalysts evolve H2 from neutral water faster than monometallic catalysts, even at lower overpotential. The unexpected origin of this improvement is non-covalent supramolecular self-assembly into nanoscale aggregates that efficiently harvest light and form H–H bonds. Monometallic catalysts containing long-chain alkane substituents leverage the self-assembly to evolve H2 from neutral water at low overpotential and with rates close to the expected maximum for this light-driven water splitting reaction. Design parameters for holding multiple catalytic sites in close proximity and tuning catalyst microenvironments emerge from this work.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and analysed in this study are included in this article and its Supplementary Information. Source data are provided with this paper.
References
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Tachibana, Y., Vayssieres, L. & Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photon. 6, 511–518 (2012).
Esswein, A. J. & Nocera, D. G. Hydrogen production by molecular photocatalysis. Chem. Rev. 107, 4022–4047 (2007).
Berardi, S. et al. Molecular artificial photosynthesis. Chem. Soc. Rev. 43, 7501–7519 (2014).
Kärkäs, M. D., Verho, O., Johnston, E. V. & Åkermark, B. Artificial photosynthesis: molecular systems for catalytic water oxidation. Chem. Rev. 114, 11863–12001 (2014).
Ashford, D. L. et al. Molecular chromophore–catalyst assemblies for solar fuel applications. Chem. Rev. 115, 13006–13049 (2015).
Brereton, K. R., Bonn, A. G. & Miller, A. J. M. Molecular photoelectrocatalysts for light-driven hydrogen production. ACS Energy Lett. 3, 1128–1136 (2018).
Reyes Cruz, E. A. et al. Molecular-modified photocathodes for applications in artificial photosynthesis and solar-to-fuel technologies. Chem. Rev. 122, 16051–16109 (2022).
Costentin, C., Dridi, H. & Savéant, J.-M. Molecular catalysis of H2 evolution: diagnosing heterolytic versus homolytic pathways. J. Am. Chem. Soc. 136, 13727–13734 (2014).
Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 42, 1995–2004 (2009).
Valdez, C. N., Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Catalytic hydrogen evolution from a covalently linked dicobaloxime. Proc. Natl Acad. Sci. USA 109, 15589–15593 (2012).
Han, Y. et al. Singly versus doubly reduced nickel porphyrins for proton reduction: experimental and theoretical evidence for a homolytic hydrogen‐evolution reaction. Angew. Chem. Int. Ed. 55, 5457–5462 (2016).
Guo, X. et al. Homolytic versus heterolytic hydrogen evolution reaction steered by a steric effect. Angew. Chem. Int. Ed. 59, 8941–8946 (2020).
Pitman, C. L. & Miller, A. J. M. Molecular photoelectrocatalysts for visible light-driven hydrogen evolution from neutral water. ACS Catal. 4, 2727–2733 (2014).
Stratakes, B. M. & Miller, A. J. M. H2 evolution at an electrochemical ‘underpotential’ with an iridium-based molecular photoelectrocatalyst. ACS Catal. 10, 9006–9018 (2020).
Rivier, L. et al. Photoproduction of hydrogen by decamethylruthenocene combined with electrochemical recycling. Angew. Chem. Int. Ed. 56, 2324–2327 (2017).
Rivier, L. et al. Mechanistic study on the photogeneration of hydrogen by decamethylruthenocene. Chem. Eur. J. 25, 12769–12779 (2019).
Huang, J., Sun, J., Wu, Y. & Turro, C. Dirhodium(II,II)/NiO photocathode for photoelectrocatalytic hydrogen evolution with red light. J. Am. Chem. Soc. 143, 1610–1617 (2021).
Chambers, M. B., Kurtz, D. A., Pitman, C. L., Brennaman, M. K. & Miller, A. J. M. Efficient photochemical dihydrogen generation initiated by a bimetallic self-quenching mechanism. J. Am. Chem. Soc. 138, 13509–13512 (2016).
Stratakes, B. M., Dempsey, J. L. & Miller, A. J. M. Determining the overpotential of electrochemical fuel synthesis mediated by molecular catalysts: recommended practices, standard reduction potentials, and challenges. ChemElectroChem 8, 4161–4180 (2021).
Dadci, L. et al. π-Arene aqua complexes of cobalt, rhodium, iridium, and ruthenium: preparation, structure, and kinetics of water exchange and water substitution. Inorg. Chem. 34, 306–315 (1995).
Pitman, C. L., Brereton, K. R. & Miller, A. J. M. Aqueous hydricity of late metal catalysts as a continuum tuned by ligands and the medium. J. Am. Chem. Soc. 138, 2252–2260 (2016).
Rountree, E. S., McCarthy, B. D., Eisenhart, T. T. & Dempsey, J. L. Evaluation of homogeneous electrocatalysts by cyclic voltammetry. Inorg. Chem. 53, 9983–10002 (2014).
Wadsworth, B. L., Beiler, A. M., Khusnutdinova, D., Reyes Cruz, E. A. & Moore, G. F. Interplay between light flux, quantum efficiency, and turnover frequency in molecular-modified photoelectrosynthetic assemblies. J. Am. Chem. Soc. 141, 15932–15941 (2019).
Nguyen, N. P., Wadsworth, B. L., Nishiori, D., Reyes Cruz, E. A. & Moore, G. F. Understanding and controlling the performance-limiting steps of catalyst-modified semiconductors. J. Phys. Chem. Lett. 12, 199–203 (2021).
Delahay, P. & Stiehl, G. L. Theory of catalytic polarographic currents. J. Am. Chem. Soc. 74, 3500–3505 (1952).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2000).
Costentin, C., Passard, G. & Savéant, J.-M. Benchmarking of homogeneous electrocatalysts: overpotential, turnover frequency, limiting turnover number. J. Am. Chem. Soc. 137, 5461–5467 (2015).
Weberg, A. B., Murphy, R. P. & Tomson, N. C. Oriented internal electrostatic fields: an emerging design element in coordination chemistry and catalysis. Chem. Sci. 13, 5432–5446 (2022).
Jiang, X. Hydrophobic-lipophilic interactions. Aggregation and self-coiling of organic molecules. Acc. Chem. Res. 21, 362–367 (1988).
Blesic, M. et al. Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem. 9, 481–490 (2007).
Keijer, T., Bouwens, T., Hessels, J. & Reek, J. N. H. Supramolecular strategies in artificial photosynthesis. Chem. Sci. 12, 50–70 (2021).
Pannwitz, A. et al. Roadmap towards solar fuel synthesis at the water interface of liposome membranes. Chem. Soc. Rev. 50, 4833–4855 (2021).
Wang, Y.-H. et al. Brønsted acid scaling relationships enable control over product selectivity from O2 reduction with a mononuclear cobalt porphyrin catalyst. ACS Cent. Sci. 5, 1024–1034 (2019).
Martin, D. J., Wise, C. F., Pegis, M. L. & Mayer, J. M. Developing scaling relationships for molecular electrocatalysis through studies of Fe-porphyrin-catalyzed O2 reduction. Acc. Chem. Res. 53, 1056–1065 (2020).
Nie, W. & McCrory, C. C. L. Strategies for breaking molecular scaling relationships for the electrochemical CO2 reduction reaction. Dalton Trans. 51, 6993–7010 (2022).
Boulas, P. L., Go, M. & Echegoyen, L. Electrochemistry of supramolecular systems. Angew. Chem. Int. Ed. 37, 216–247 (1998).
Wiester, M. J., Ulmann, P. A. & Mirkin, C. A. Enzyme mimics based upon supramolecular coordination chemistry. Angew. Chem. Int. Ed. 50, 114–137 (2011).
Raynal, M., Ballester, P., Vidal-Ferran, A. & van Leeuwen, P. W. N. M. Supramolecular catalysis. Part 1: non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 43, 1660–1733 (2014).
Raynal, M., Ballester, P., Vidal-Ferran, A. & van Leeuwen, P. W. N. M. Supramolecular catalysis. Part 2: artificial enzyme mimics. Chem. Soc. Rev. 43, 1734–1787 (2014).
Zeng, T. et al. Hybrid bilayer membranes as platforms for biomimicry and catalysis. Nat. Rev. Chem. 6, 862–880 (2022).
Bonchio, M. et al. Hierarchical organization of perylene bisimides and polyoxometalates for photo-assisted water oxidation. Nat. Chem. 11, 146–153 (2019).
Nguyen, H. D., Jana, R. D., Campbell, D. T., Tran, T. V. & Do, L. H. Lewis acid-driven self-assembly of diiridium macrocyclic catalysts imparts substrate selectivity and glutathione tolerance. Chem. Sci. 14, 10264–10272 (2023).
Yu, J. et al. Artificial spherical chromatophore nanomicelles for selective CO2 reduction in water. Nat. Catal. 6, 464–475 (2023).
Yang, B. et al. Self-assembled amphiphilic water oxidation catalysts: control of O−O bond formation pathways by different aggregation patterns. Angew. Chem. Int. Ed. 55, 6229–6234 (2016).
White, C., Yates, A., Maitlis, P. M. & Heinekey, D. M. (η5-Pentamethylcyclopentadienyl)rhodium and -iridium compounds. In Inorganic Syntheses Vol. 29 (ed. Grimes, R. N.) 228–234 (Wiley, 1992).
Himeda, Y., Onozawa-Komatsuzaki, N., Sugihara, H. & Kasuga, K. Simultaneous tuning of activity and water solubility of complex catalysts by acid-base equilibrium of ligands for conversion of carbon dioxide. Organometallics 26, 702–712 (2007).
Schmehl, R. H. et al. Formation and photophysical properties of iron-ruthenium tetranuclear bipyridyl complexes of the type {[(bpy)2Ru(L-L)]3Fe}. Inorg. Chem. 25, 2440–2445 (1986).
Furue, M. et al. Intramolecular energy transfer in covalently linked polypyridine ruthenium(II)/osmium(II) binuclear complexes. Ru(II)(bpy)2Mebpy– (CH2)n– MebpyOs(II)(bpy)2 (n = 2, 3, 5, and 7). Bull. Chem. Soc. Jpn 64, 1632–1640 (1991).
Mulyana, Y. et al. Oligonuclear polypyridylruthenium(ii) complexes incorporating flexible polar and non-polar bridges: synthesis, DNA-binding and cytotoxicity. Dalton Trans. 40, 1510–1523 (2011).
Kühn, F. E. et al. Octahedral bipyridine and bipyrimidine dioxomolybdenum(VI) complexes: characterization, application in catalytic epoxidation, and density functional mechanistic study. Chem. Eur. J. 8, 2370–2383 (2002).
Griggs, C. G. & Smith, D. J. H. An improved synthesis of amphiphilic 4,4′-disubstituted 2,2′-bipyridines. J. Chem. Soc. Perkin Trans. 1 https://doi.org/10.1039/P19820003041 (1982).
Rubinson, K. A. Practical corrections for p(H,D) measurements in mixed H2O/D2O biological buffers. Anal. Methods 9, 2744–2750 (2017).
Krȩżel, A. & Bal, W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 98, 161–166 (2004).
Fulmer, G. R. et al. NMR chemical shifts of trace impurities: common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 29, 2176–2179 (2010).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under award DE-SC0014255 (principal investigator, A.J.M.M.). The NMR spectroscopy was supported by the National Science Foundation under grant CHE-1828183. The mass spectrometry was supported by the National Science Foundation under grant CHE-1726291. We acknowledge N. Kumarage for assistance with NMR spectral acquisition. We also acknowledge A. Tripathy, Director of the Macromolecular Interactions Facility where the DLS experiments were performed, for experimental assistance. The DLS experimental work was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA016086. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge A. Kumbhar for assistance with TEM imaging. The TEM work was performed at the Chapel Hill Analytical and Nanofabrication Laboratory, CHANL, a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, grant ECCS-1542015, as part of the National Nanotechnology Coordinated Infrastructure, NNCI.
Author information
Authors and Affiliations
Contributions
T.L., J.R., A.G.B., M.B.C., C.L.P. and T.J. synthesized and characterized the iridium catalysts. I.N.C., T.L. and A.G.B. performed the CV studies. I.N.C., T.L., J.R. and T.J. conducted the CA and CPE studies. I.N.C., T.L. and M.A.t.H. analysed the catalyst aggregates using light scattering and NMR methods. A.J.M.M. conceived of the idea, directed the project and supervised the experimental design, execution and interpretation. I.N.C., T.L. and A.J.M.M. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–134 and Tables 1–4.
Supplementary Data 1
CA source data.
Supplementary Data 2
CV source data.
Supplementary Data 3
DLS source data.
Supplementary Data 4
UV–visible spectroscopy source data.
Source data
Source Data Fig. 3
CV and CA data.
Source Data Fig. 4
DLS data.
Source Data Fig. 5
CV and DLS data.
Source Data Fig. 6
CA source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cloward, I.N., Liu, T., Rose, J. et al. Catalyst self-assembly accelerates bimetallic light-driven electrocatalytic H2 evolution in water. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01483-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01483-3
This article is cited by
-
Assembling the pieces to improve catalysis
Nature Chemistry (2024)