Abstract
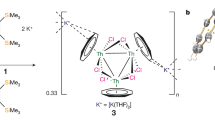
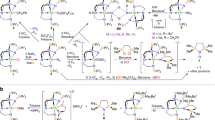
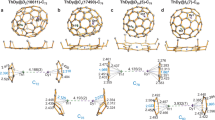
There is continued burgeoning interest in metal–metal multiple bonding to further our understanding of chemical bonding across the periodic table. However, although polar covalent metal–metal multiple bonding is well known for the d and p blocks, it is relatively underdeveloped for actinides. Homometallic examples are found in spectroscopic or fullerene-confined species, and heterometallic variants exhibiting a polar covalent σ bond supplemented by up to two dative π bonds are more prevalent. Hence, securing polar covalent actinide double and triple metal–metal bonds under normal experimental conditions has been a fundamental target. Here we exploit the protonolysis and dehydrocoupling chemistry of the parent dihydrogen-antimonide anion, to report one-, two- and three-fold thorium–antimony bonds, thus introducing polar covalent actinide–metal multiple bonding under normal experimental conditions between some of the heaviest ions in the periodic table with little or no bulky-substituent protection at the antimony centre. This provides fundamental insights into heavy element multiple bonding, in particular the tension between orbital-energy-driven and overlap-driven covalency for the actinides in a relativistic regime.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited with the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2285677 (5), 2285678 (6), 2285679 (7), 2285680 (8), 2285681 (9) and 2285682 (10). These data can be obtained free of charge from CCDC via www.ccdc.cam.ac.uk/data_request/cif. All other data are presented in the main text and the Supplementary Information, and are also available from the corresponding authors on reasonable request.
References
Cotton, F. A., Murillo, C. A. & Walton, R. A. (eds) Multiple Bonds Between Metal Atoms 3rd edn (Springer, 2005).
Liddle. S. T. (ed.) Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties (Wiley, 2015).
Patel, D. & Liddle, S. T. f-element-metal bond chemistry. Rev. Inorg. Chem. 32, 1–22 (2012).
Fang, W., Maron, L. & Zhu, C. in Handbook on the Physics and Chemistry of Rare Earths Vol. 63 (eds Bünzli, J.-C. G. & Kauzlarich, S. M.) Ch. 327 (North-Holland/Elsevier, 2023).
Gorokhov, L. N., Emelyanov, A. M. & Khodeev, Y. S. Mass-spectroscopic investigation of stability of gaseous U2O2 and U2. High Temp. 12, 1307–1309 (1974).
Steimle, T., Kokkin, D. L., Muscarella, S. & Ma, T. Detection of the thorium dimer via two-dimensional fluorescence spectroscopy. J. Phys. Chem. A 119, 9281–9285 (2015).
Chi, C. et al. Preparation and characterization of uranium-iron triple-bonded UFe(CO)3− and OUFe(CO)3− complexes. Angew. Chem. Int. Ed. 56, 6932–6936 (2017).
Souter, P. F., Kushto, G. P. & Andrews, L. IR spectra of uranium hydride molecules isolated in solid argon. Chem. Commun. 1996, 2401–2402 (1996).
Souter, P. F., Kushto, G. P., Andrews, L. & Neurock, M. Experimental and theoretical evidence for the formation of several uranium hydride molecules. J. Am. Chem. Soc. 119, 1682–1687 (1997).
Zhang, X. et al. U2@Ih(7)-C80: crystallographic characterization of a long-sought dimetallic actinide endohedral fullerene. J. Am. Chem. Soc. 140, 3907–3915 (2018).
Zhuang, J. et al. Characterization of a strong covalent Th3+-Th3+ bond inside an Ih(7)-C80 fullerene cage. Nat. Commun. 12, 2372 (2021).
Ritchey, J. M. et al. An organothorium-nickel phosphido complex with a short thorium-nickel distance. The structure of Th(η5-C5Me5)2(μ-PPh2)2Ni(CO)2. J. Am. Chem. Soc. 107, 501–503 (1985).
Sternal, R. S., Brock, C. P. & Marks, T. J. Metal-metal bonds involving actinides. Synthesis and characterization of a complex having an unsupported actinide to transition metal bond. J. Am. Chem. Soc. 107, 8270–8272 (1985).
Hay, P. J., Ryan, R. R., Salazar, K. V., Wrobleski, D. A. & Sattelberger, A. P. Synthesis and X-ray structure of (C5Me5)2Th(μ-PPh2)2Pt(PMe3): a complex with a thorium–platinum bond. J. Am. Chem. Soc. 108, 313–315 (1986).
Porchia, M. et al. Synthesis and crystal structure of triscyclopentadienyl(triphenyltin)uranium. The first example of a uranium tin bond. Chem. Commun. 1034–1035 (1986).
Bucaille, A., Le Borgne, T., Ephritikhine, M. & Daran, J.-C. Synthesis and X-ray crystal structure of a urana[1]ferrocenophane, the first tris(1,1’-ferrocenylene) metal compound. Organometallics 19, 4912–4914 (2000).
Diaconescu, P. L., Odom, A. L., Agapie, T. & Cummins, C. C. Uranium-group 14 element single bonds: isolation and characterization of a uranium(IV) silyl species. Organometallics 20, 4993–4995 (2001).
Monreal, M. J., Carver, C. T. & Diaconescu, P. L. Redox processes in a uranium bis(1,1′-diamidoferrocene) complex. Inorg. Chem. 46, 7226–7228 (2007).
Monreal, M. J. & Diaconescu, P. L. A weak interaction between iron and uranium in uranium alkyl complexes supported by cerrocene diamide ligands. Organometallics 27, 1702–1706 (2008).
Minasian, S. G., Krinsky, J. L., Williams, V. A. & Arnold, J. A heterobimetallic complex with an unsupported uranium(III)-aluminum(I) bond: (CpSiMe3)3U-AlCp* (Cp* = C5Me5). J. Am. Chem. Soc. 130, 10086–10087 (2008).
Liddle, S. T. et al. σ and π donation in an unsupported uranium-gallium bond. Angew. Chem. Int. Ed. 48, 1077–1080 (2009).
Gardner, B. M. McMaster, J., Lewis, W. & Liddle, S. T. Synthesis and structure of [{N(CH2CH2NSiMe3)3}URe(η5-C5H5)2]: a heterobimetallic complex with an unsupported uranium-rhenium bond. Chem. Commun. 2009, 2851–2853 (2009).
Minasian, S. G. et al. A comparison of 4ƒ vs 5ƒ metal-metal bonds in (CpSiMe3)3M-ECp* (M = Nd, U; E;= Al, Ga; Cp* = C5Me5): synthesis, thermodynamics, magnetism, and electronic structure. J. Am. Chem. Soc. 131, 13767–13783 (2009).
Broderick, E. M., Gutzwiller, N. P. & Diaconescu, P. L. Inter- and intramolecular hydroamination with a uranium dialkyl precursor. Organometallics 29, 3242–3251 (2010).
Patel, D. et al. Structural and theoretical insights into the perturbation of uranium-rhenium bonds by dative Lewis base ancillary ligands. Chem. Commun. 47, 295–297 (2011).
Patel, D. et al. A formal high oxidation state inverse-sandwich diuranium complex: a new route to f-block-metal bonds. Angew. Chem. Int. Ed. 50, 10388–10392 (2011).
Gardner, B. M. et al. An unsupported uranium-rhenium complex prepared by alkane elimination. Chem. Eur. J. 17, 6909–6912 (2011).
Gardner, B. M. et al. The nature of unsupported uranium-ruthenium bonds: a combined experimental and theoretical study. Chem. Eur. J. 17, 11266–11273 (2011).
Monreal, M. J., Khan, S. I., Kiplinger, J. L. & Diaconescu, P. L. Molecular quadrangle formation from a diuranium μ-η6,η6-toluene complex. Chem. Commun. 47, 9119–9121 (2011).
Fortier, S., Walensky, J. R., Wu, G. & Hayton, T. W. High-valent uranium alkyls: evidence for the formation of UVI(CH2SiMe3)6. J. Am. Chem. Soc. 133, 11732–11743 (2011).
Napoline, J. W. et al. Tris(phosphinoamide)-supported uranium-cobalt heterobimetallic complexes featuring Co → U dative interactions. Inorg. Chem. 52, 12170–12177 (2013).
Duhovic, S. et al. Investigation of the electronic structure of mono(1,1′-diamidoferrocene) uranium(IV) complexes. Organometallics 32, 6012–6021 (2013).
Ward, A. L., Lukens, W. W., Lu, C. C. & Arnold, J. Photochemical route to actinide-transition metal bonds: synthesis, characterization and reactivity of a series of thorium and uranium heterobimetallic complexes. J. Am. Chem. Soc. 136, 3647–3654 (2014).
Hlina, J. A., Pankhurst, J. R., Kaltsoyannis, N. & Arnold, P. L. Metal-metal bonding in uranium-group 10 complexes. J. Am. Chem. Soc. 138, 3333–3345 (2016).
Winston, M. S., Batista, E. R., Yang, P., Tondreau, A. M. & Boncella, J. M. Extending stannyl anion chemistry to the actinides: synthesis and characterization of a uranium-tin bond. Inorg. Chem. 55, 5534–5539 (2016).
Lichtenberger, N. et al. Main group metal-actinide magnetic coupling and structural response upon U4+ inclusion into Bi, Tl/Bi, or Pb/Bi cages. J. Am. Chem. Soc. 138, 9033–9036 (2016).
Yang, P. et al. Experimental and computational studies on the formation of thorium-copper heterobimetallics. Chem. Eur. J. 22, 13845–13849 (2016).
Hlina, J. A., Wells, J. A. L., Pankhurst, J. R., Love, J. B. & Arnold, P. L. Uranium rhodium bonding in heterometallic complexes. Dalton Trans. 46, 5540–5545 (2017).
Fortier, S. et al. An N-tethered uranium(III) arene complex and the synthesis of an unsupported U-Fe bond. Organometallics 36, 4591–4599 (2017).
Camp, C., Toniolo, D., Andrez, J., Pécaut, J. & Mazzanti, M. A versatile route to homo- and hetero-bimetallic 5ƒ–5ƒ and 3d–5ƒ complexes supported by a redox active ligand framework. Dalton Trans. 46, 11145–11148 (2017).
Rookes, T. M. et al. Actinide-pnictide (An-Pn) bonds spanning non-metal, metalloid, and metal combinations (An = U, Th; Pn = P, As, Sb, Bi). Angew. Chem. Int. Ed. 57, 1332–1336 (2018).
Lu, E., Wooles, A. J., Gregson, M., Cobb, P. J. & Liddle, S. T. A very short uranium(IV)-rhodium(I) bond with net double-dative bonding character. Angew. Chem. Int. Ed. 57, 6587–6591 (2018).
Ayres, A. J. et al. Actinide-transition metal bonding in heterobimetallic uranium- and thorium-molybdenum paddlewheel complexes. Chem. Commun. 54, 13515–13518 (2018).
Feng, G. et al. Identification of a uranium-rhodium triple bond in a heterometallic cluster. Proc. Natl Acad. Sci. USA 116, 17654–17658 (2019).
Feng, G. et al. Transition-metal-bridged bimetallic clusters with multiple uranium-metal bonds. Nat. Chem. 11, 248–253 (2019).
Feng, G., McCabe, K. N., Wang, S., Maron, L. & Zhu, C. Construction of heterometallic clusters with multiple uranium-metal bonds by using dianionic nitrogen-phosphorus ligands. Chem. Sci. 11, 7585–7592 (2020).
Xin, X. et al. Dinitrogen cleavage by a heterometallic cluster featuring multiple uranium-rhodium bonds. J. Am. Chem. Soc. 142, 15004–15011 (2020).
Eulenstein, A. R. et al. Substantial π-aromaticity in the anionic heavy-metal cluster [Th@Bi12]4−. Nat. Chem. 13, 149–155 (2021).
Tarlton, M. L., Kelley, S. P. & Walensky, J. R. Crystal structures of metallocene complexes with uranium-germanium bonds. Acta Crystallogr. E 77, 1258–1262 (2021).
Boronski, J. T. et al. A crystalline tri-thorium cluster with σ-aromatic metal-metal bonding. Nature 598, 72–75 (2021).
Wang, P. et al. Selective hydroboration of terminal alkynes catalysed by heterometallic clusters with uranium-metal triple bonds. Chem 8, 1361–1375 (2022).
Barluzzi, L. et al. Heterometallic uranium/molybdenum nitride synthesis via partial N-atom transfer. Chem. Commun. 58, 4655–4658 (2022).
Beuthert, K., Weinert, B., Wilson, R. J., Weigend, F. & Dehnen, S. [M@Sn14−xSbx]q− (M = La, Ce, or U; x = 6-8; q = 3, 4): Interaction of 4ƒ or 5ƒ metal ions with 5p metal atoms in intermetalloid clusters. Inorg. Chem. 62, 1885–1890 (2023).
Shen, J. et al. Complexes featuring a cis-[M⇉U⇇M] core (M = Rh, Ir): a new route to uranium-metal multiple bonds. Angew. Chem. Int. Ed. 62, e202303379 (2023).
Ye, C. Z. et al. A versatile strategy for the formation of hydride-bridged actinide-iridium multimetallics. Chem. Sci. 14, 861–868 (2023).
Li, K. et al. Heterotrimetallic clusters with U-Ni-Ge and U-Ni-Sn units. Polyhedron 243, 116548 (2023).
Hayton, T. W. Recent developments in actinide-ligand multiple bonding. Chem. Commun. 49, 2956–2973 (2013).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Rudel, S. S., Deubner, H. L., Müller, M., Karttunen, A. J. & Kraus, F. Complexes featuring a linear [N≡U≡N] core isoelectronic to the uranyl cation. Nat. Chem. 12, 962–967 (2020).
Du, J., Cobb, P. J., Ding, J., Mills, D. P. & Liddle, S. T. f-Element heavy pnictogen chemistry. Chem. Sci. 15, 13–45 (2024).
Gardner, B. M. et al. Triamidoamine-uranium(IV)-stabilized terminal parent phosphide and phosphinidene complexes. Angew. Chem. Int. Ed. 53, 4484–4488 (2014).
Wildman, E. P., Balázs, G., Wooles, A. J., Scheer, M. & Liddle, S. T. Thorium–phosphorus triamidoamine complexes containing Th–P single- and multiple-bond interactions. Nat. Commun. 7, 12884 (2016).
Du, J. et al. Actinide pnictinidene chemistry: a terminal thorium parent-arsinidene complex stabilised by a super-bulky triamidoamine ligand. Angew. Chem. Int. Ed. 61, e202211627 (2022).
Gardner, B. M. et al. Triamidoamine uranium(IV)–arsenic complexes containing one-, two-, and three-fold U–As bonding interactions. Nat. Chem. 7, 582–590 (2015).
Wildman, E. P., Balázs, G., Wooles, A. J., Scheer, M. & Liddle, S. T. Triamidoamine thorium-arsenic complexes with parent arsenide, arsinidiide and arsenido structural motifs. Nat. Commun. 8, 14769 (2017).
Magnall, R. Photolytic and reductive activations of 2-arsaethynolate in a uranium–triamidoamine complex: decarbonylative arsenic-group transfer reactions and trapping of a highly bent and reduced form. Chem. Eur. J. 25, 14246–14252 (2019).
Rookes, T. M. et al. Crystalline diuranium phosphinidiide and μ-phosphido complexes with symmetric and asymmetric cores. Angew. Chem. Int. Ed. 56, 10495–10500 (2017).
Dollberg, K., Schneider, S., Richter, R.-M., Dunaj, T. & Von Hänisch, C. Synthesis and application of alkali metal antimonide – a new approach to antimony chemistry. Angew. Chem. Int. Ed. 61, e202213098 (2022).
Sasamori, T., Takeda, N. & Tokitoh, N. Synthesis of a stable stibabismuthene; the first compound with an antimony-bismuth double bond. Chem. Commun. 1353–1354 (2000).
Helling, C., Wölper, C., Schulte, Y., Cutsail, G. E. III & Schulz, S. Synthesis of a Ga-stabilized As-centered radical and a gallastibene by tailoring group 15 element-carbon bond strengths. Inorg. Chem. 58, 10323–10332 (2019).
Bringewski, F., Huttner, G. & Imhof, W.Stibacumulenium-ionen: Darstellung und struktur von [η6-Me6C6(CO)2Cr=Sb=Cr(CO)2-η6-C6Me6]+. J. Organomet. Chem. 448, C3–C5 (1993).
Scheer, M., Müller, J., Baum, G. & Häser, M. Antimony as a symmetrically bridged ligand in a novel neutral complex. Chem. Commun. 1998, 2505–2506 (1998).
Balázs, G., Sierka, M. & Scheer, M. Antimony-tungsten triple bond: a stable complex with a terminal antimony ligand. Angew. Chem. Int. Ed. 44, 4920–4924 (2005).
Krüger, J., Ganesamoorthy, C., John, L., Wölper, C. & Schulz, S. A general pathway for the synthesis of gallastibenes containing Ga=Sb double bonds. Chem. Eur. J. 24, 9157–9164 (2018).
Ganesamoorthy, C. et al. From stable Sb- and Bi-centered radicals to a compound with a Ga=Sb double bond. Nat. Commun. 9, 87 (2018).
Helling, C. et al. A mechanistic study on reactions of group 13 diyls LM with Cp*SbX2: from stibanyl radicals to antimony hydrides. Chem. Eur. J. 26, 13390–13339 (2020).
Krüger, J., Wölper, C. & Schulz, S. From π-bonded gallapnictenes to nucleophilic, redox-active metal-coordinated pnictanides. Angew. Chem. Int. Ed. 60, 3572–3575 (2021).
Gardner, B. M. et al. Assessing crystal field and magnetic interactions in diuranium-μ-chalcogenide triamidoamine complexes with UIV-E-UIV cores (E = S, Se, Te): implications for determining the presence or absence of actinide-actinide magnetic exchange. Chem. Sci. 8, 6207–6217 (2017).
Gardner, B. M. et al. Isolation of elusive HAsAsH in a crystalline diuranium(IV) complex. Angew. Chem. Int. Ed. 54, 15250–15254 (2015).
Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J. Phys. Chem. A 119, 2326–2337 (2015).
Schneider, S., Ivlev, S. & Von Hänisch, C. Stibine as a reagent in molecular chemistry – target synthesis of primary and secondary stibanyl-gallanes and their lighter homologues. Chem. Commun. 57, 3781–3784 (2021).
Cherng, J.-J., Lee, G.-H., Peng, S.-M., Ueng, C.-H. & Shieh, M. Synthesis and characterization of the new series of chromium−group 15 hydride complexes [Et4N]2[HE{Cr(CO)5}3] (E = As, Sb). Organometallics 19, 213–215 (2000).
Brown, J. L., Fortier, S., Lewis, R. A., Wu, G. & Hayton, T. W. A complete family of terminal uranium chalcogenides, [U(E)(N{SiMe3}2)3]− (E = O, S, Se, Te). J. Am. Chem. Soc. 134, 15468–15475 (2012).
Smiles, D. E., Wu, G., Hrobárik, P. & Hayton, T. W. Use of 77Se and 125Te NMR spectroscopy to probe covalency of the actinide-chalcogen bonding in [Th(En){N(SiMe3)2}3]− (E = Se, Te; n = 1,2) and their oxo-uranium(VI) congeners. J. Am. Chem. Soc. 138, 814–825 (2016).
Schoo, C. et al. Samarium polystibides derived from highly activated nanoscale antimony. Angew. Chem. Int. Ed. 57, 5912–5916 (2018).
Bader, R. F. W., Slee, T. S., Cremer, D. & Kraka, E. Description of conjugation and hyperconjugation in terms of electron distributions. J. Am. Chem. Soc. 105, 5061–5068 (1983).
Filippou, A. C., Weidemann, N., Schnakenburg, G., Rohde, H. & Philippopoulos, A. I. Tungsten–lead triple bonds: syntheses, structures, and coordination chemistry of the plumbylidyne complexes trans-[X(PMe3)4W≡Pb(2,6-Trip2C6H3)]. Angew. Chem. Int. Ed. 43, 6512–6516 (2004).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Kaltsoyannis, N. Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg. Chem. 52, 3407–3413 (2013).
Barker, B. J. & Sears, P. G. Conductance behavior of some ammonium and partially substituted ammonium tetraphenylborates in 3-methyl-2-oxazolidone and 3-tert-butyl-2-oxazolidone at 25°. J. Phys. Chem. 78, 2687–2688 (1974).
Gardner, B. M. et al. The role of 5f-orbital participation in unexpected inversion of the σ-bond metathesis reactivity trend of triamidoamine thorium(IV) and uranium(IV) alkyls. Chem. Sci. 5, 2489–2497 (2014).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
CrysAlis PRO v.1.171.40.69a (Oxford Diffraction/Agilent Technologies, 2020).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45, 849–854 (2012).
Persistence of Vision Raytracer v.6.2 (Persistence of Vision Pty, 2005).
Hitchcock, P. B., Lappert, M. F., Maron, L. & Protchenko, A. V. Lanthanum does form stable molecular compounds in the +2 oxidation state. Angew. Chem. Int. Ed. 47, 1488–1491 (2008).
Gardner, B. M. et al. Assessing crystal field and magnetic interactions in diuranium-μ-chalcogenide triamidoamine complexes with UIV-E-UIV cores (E = S, Se, Te): implications for determining the presence or absence of actinide-actinide magnetic exchange. Chem. Sci. 8, 6207–6217 (2017).
Fonseca Guerra, A. C., Snijders, J. G., Te Velde, G. & Baerends, E. J. Towards an order-N DFT method. Theor. Chem. Acc. 99, 391–403 (1998).
Te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Van Lenthe, E., Baerends, E. J. & Snijders, J. G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 99, 4597–4610 (1993).
Van Lenthe, E., Baerends, E. J. & Snijders, J. G. Relativistic total energy using regular approximations. J. Chem. Phys. 101, 9783–9792 (1994).
Van Lenthe, E., Ehlers, A. E. & Baerends, E. J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 110, 8943–8953 (1999).
Vosko, S. H., Wilk, L. & Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 58, 1200–1211 (1980).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 38, 3098–3100 (1988).
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986).
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, 1990).
Bader, R. F. W. A bond path: a universal indicator of bonded interactions. J. Phys. Chem. A 102, 7314–7323 (1998).
Glendening, E. D., Landis, C. R. & Weinhold, F. NBO 6.0: Natural Bond Orbital Analysis Program. J. Comput. Chem. 34, 2134–2134 (2013).
Motta, L. C. & Autschbach, J. Actinide inverse trans influence versus cooperative pushing from below and multi-center bonding. Nat. Commun. 14, 4307 (2023).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (grants EP/T011289/1, EP/P001386/1, EP/M027015/1, and EP/W029057/1, S.T.L.), European Research Council (CoG612724, S.T.L.), Deutsche Forschungsgemeinschaft (HA 3466/11-1, C.v.H.), Philipps-Universität Marburg (K.D., C.v.H.) and the University of Manchester including computational resources and associated support services from the Computational Shared Facility (J.D., J.A.S., A.J.W., S.T.L.). The Alexander von Humboldt Foundation is thanked for a Friedrich Wilhelm Bessel Research Award (S.T.L.). We thank M. Jennings and A. Davies at the University of Manchester for CHN microanalyses.
Author information
Authors and Affiliations
Contributions
J.D. synthesized the thorium–antimony complexes and characterized them. K.D. prepared the antimony reagent. J.A.S. recorded the optical data and fitted them to the TD-DFT calculations. A.J.W. collected and refined the crystallographic data. S.T.L. conducted the quantum chemical calculations. C.v.H. and S.T.L. conceived the research idea, directed the research, analysed and interpreted all the data, and wrote the manuscript, with contributions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Crystallographic alerts and justifications, Supplementary Figs. 1–70 and Tables 1–10.
Supplementary Data 1
Cif data for 5 including fcf.
Supplementary Data 2
Cif data for 6 including fcf.
Supplementary Data 3
Cif data for 7 including fcf.
Supplementary Data 4
Cif data for 8 including fcf.
Supplementary Data 5
Cif data for 9 including fcf.
Supplementary Data 6
Cif data for 10 including fcf.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, J., Dollberg, K., Seed, J.A. et al. Thorium(iv)–antimony complexes exhibiting single, double, and triple polar covalent metal–metal bonds. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01448-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01448-6