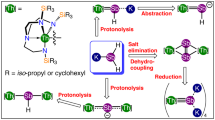
Abstract
Metal–metal bonding is a widely studied area of chemistry1,2,3, and has become a mature field spanning numerous d transition metal and main group complexes4,5,6,7. By contrast, actinide–actinide bonding, which is predicted to be weak8, is currently restricted to spectroscopically detected gas-phase U2 and Th2 (refs. 9,10), U2H2 and U2H4 in frozen matrices at 6–7 K (refs. 11,12), or fullerene-encapsulated U2 (ref. 13). Furthermore, attempts to prepare thorium–thorium bonds in frozen matrices have produced only ThHn (n = 1–4)14. Thus, there are no isolable actinide–actinide bonds under normal conditions. Computational investigations have explored the probable nature of actinide–actinide bonding15, concentrating on localized σ-, π-, and δ-bonding models paralleling d transition metal analogues, but predictions in relativistic regimes are challenging and have remained experimentally unverified. Here, we report thorium–thorium bonding in a crystalline cluster, prepared and isolated under normal experimental conditions. The cluster exhibits a diamagnetic, closed-shell singlet ground state with a valence-delocalized three-centre-two-electron σ-aromatic bond16,17 that is counter to the focus of previous theoretical predictions. The experimental discovery of actinide σ-aromatic bonding adds to main group and d transition metal analogues, extending delocalized σ-aromatic bonding to the heaviest elements in the periodic table and to principal quantum number six, and constitutes a new approach to elaborate actinide–actinide bonding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
X-ray data are available free of charge from the Cambridge Crystallographic Data Centre under reference 2061981. Methods (general considerations, starting materials, experimental, crystallographic, spectroscopic, magnetic, computational data and refs. 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88), Extended Data Figs. 1–9, Extended Data Tables 1,2 and Supplementary Tables 1–7) can be found online. All other data are available from S.T.L. on reasonable request.
References
Cotton, F. A., Murillo, C. A. & Walton, R. A. (eds) Multiple bonds between metal atoms 3rd edn (Springer-Verlag, 2005).
Parkin, G. (ed.) Metal-metal bonding (Springer-Verlag, 2010).
Liddle, S. T. (ed.) Molecular metal-metal bonds: compounds, synthesis, properties (Wiley-VCH, 2015).
Wagner, F. R., Noor, A. & Kempe, R. Ultrashort metal–metal distances and extreme bond orders. Nat. Chem. 1, 529–536 (2009).
Jones, C. Dimeric magnesium(I) β-diketiminates: a new class of quasi-universal reducing agent. Nat. Rev. Chem. 1, 0059 (2017).
Popov, I. A., Starikova, A. A., Steglenko, D. V. & Boldyrev, A. I. Usefulness of the σ‐aromaticity and σ‐antiaromaticity concepts for clusters and solid‐state compounds. Chem. Eur. J. 24, 292–305 (2018).
Chipman, J. A. & Berry, J. F. Paramagnetic metal–metal bonded heterometallic complexes. Chem. Rev. 120, 2409–2447 (2020).
Cavigliasso, G. & Kaltsoyannis, N. On the paucity of molecular actinide complexes with unsupported metal−metal bonds: a comparative investigation of the electronic structure and metal−metal bonding in U2X6 (X = Cl, F, OH, NH2, CH3) complexes and d-block analogues. Inorg. Chem. 45, 6828–6839 (2006).
Gorokhov, L. N., Emelyanov, A. M. & Khodeev, Y. S. Mass-spectroscopic investigation of stability of gaseous U2O2 and U2. High Temp. 12, 1307–1309 (1974).
Steimle, T., Kokkin, D. L., Muscarella, S. & Ma, T. Detection of the thorium dimer via two-dimensional fluorescence spectroscopy. J. Phys. Chem. A 119, 9281–9285 (2015).
Souter, P. F., Kushto, G. P. & Andrews, L. IR spectra of uranium hydride molecules isolated in solid argon. Chem. Commun. 2401–2402 (1996).
Souter, P. F., Kushto, G. P., Andrews, L. & Neurock, M. Experimental and theoretical evidence for the formation of several uranium hydride molecules. J. Am. Chem. Soc. 119, 1682–1687 (1997).
Zhang, X. et al. U2@Ih(7)-C80: crystallographic characterization of a long-sought dimetallic actinide endohedral fullerene. J. Am. Chem. Soc. 140, 3907–3915 (2018).
Souter, P. F., Kushto, G. P., Andrews, L. & Neurock, M. Experimental and theoretical evidence for the isolation of thorium hydride molecules in argon matrices. J. Phys. Chem. A 101, 1287–1291 (1997).
Knecht, S., Jensen, H. J. A. & Saue, T. Relativistic quantum chemical calculations show that the uranium molecule U2 has a quadruple bond. Nat. Chem. 11, 40–44 (2019).
Chen, Z., Wannere, C. S., Corminboeuf, C., Puchta, R. & Schleyer, P. von R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105, 3842–3888 (2005).
Tsipis, C. A. Aromaticity/antiaromaticity in “bare” and “ligand-stabilized” rings of metal atoms. Struct. Bond. 136, 217–274 (2010).
Boronski, J. T., Wooles, A. J. & Liddle, S. T. Heteroleptic actinocenes: a thorium(IV)–cyclobutadienyl–cyclooctatetraenyl–di-potassium-cyclooctatetraenyl complex. Chem. Sci. 11, 6789–6794 (2020).
Le Vanda, C., Solar, J. P. & Streitwieser, A. Half-sandwich cyclooctatetraenethorium compounds. J. Am. Chem. Soc. 102, 2128–2136 (1980).
Parry, J. S., Cloke, F. G. N., Coles, S. J. & Hursthouse, M. B. Synthesis and characterization of the first sandwich complex of trivalent thorium: a structural comparison with the uranium analogue. J. Am. Chem. Soc. 121, 6867–6871 (1999).
Avdeef, A., Raymond, K. N., Hodgson, K. O. & Zalkin, A. Two isostructural actinide π complexes. Crystal and molecular structure of bis(cyclooctatetraenyl)uranium(IV), U(C8H8)2, and bis(cyclooctatetraenyl)thorium(IV), Th(C8H8)2. Inorg. Chem. 11, 1083–1088 (1972).
Ortu, F., Formanuik, A., Innes, J. R. & Mills, D. P. New vistas in the molecular chemistry of thorium: low oxidation state complexes. Dalton Trans. 45, 7537–7549 (2016).
Evans, W. J. Tutorial on the role of cyclopentadienyl ligands in the discovery of molecular complexes of the rare-earth and actinide metals in new oxidation states. Organometallics 35, 3088–3100 (2016).
Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J. Phys. Chem. A 119, 2326–2337 (2015).
Huh, D. N., Roy, S., Ziller, J. W., Furche, F. & Evans, W. J. Isolation of a square-planar Th(III) complex: synthesis and structure of [Th(OC6H2tBu2-2,6-Me-4)4]1–. J. Am. Chem. Soc. 141, 12458–12463 (2019).
Old, J., Danopoulos, A. & Winston, S. Uranium complexes with dianionic O-methylated calix[4]arene ligands. New J. Chem. 27, 672–674 (2003).
Evans, W. J., Miller, K. A., Ziller, J. W. & Greaves, J. Analysis of uranium azide and nitride complexes by atmospheric pressure chemical ionization mass spectrometry. Inorg. Chem. 46, 8008–8018 (2007).
Larch, C. P., Cloke, F. G. N. & Hitchcock, P. B. Activation and reduction of diethyl ether by low valent uranium: formation of the trimetallic, mixed valence uranium oxo species [U(CpRR′)(μ-I)2]3(μ3-O) (CpRR′ = C5Me5, C5Me4H, C5H4SiMe3). Chem. Commun. 82–84 (2008).
Boronski, J. T., Doyle, L. R., Wooles, A. J., Seed, J. A. & Liddle, S. T. Synthesis and characterization of an oxo-centered homotrimetallic uranium(IV)–cyclobutadienyl dianion complex. Organometallics 39, 1824–1831 (2020).
Zhang, C., Hou, G., Zi, G., Ding, W. & Walter, M. D. An alkali-metal halide-bridged actinide phosphinidiide complex. Inorg. Chem. 58, 1571–1590 (2019).
Langeslay, R. R., Fieser, M. E., Ziller, J. W., Furche, F. & Evans, W. J. Expanding thorium hydride chemistry through Th2+, including the synthesis of a mixed-valent Th4+/Th3+ hydride complex. J. Am. Chem. Soc. 138, 4036–4045 (2016).
Clark, D. L., Grumbine, S. K., Scott, B. L. & Watkin, J. G. Unique molecular structure of the actinide hydrido aryloxide complex Th3(μ3-H)2(μ2-H)4(O-2,6-t-Bu2C6H3)6. J. Am. Chem. Soc. 117, 9089–9090 (1995).
Blanchard, S. et al. Synthesis of triangular tripalladium cations as noble‐metal analogues of the cyclopropenyl cation. Angew. Chem. Int. Ed. 53, 1987–1991 (2014).
King, R. B. Metal cluster topology. 21. Sigma aromaticity in triangular metal carbonyl clusters. Inorg. Chim. Acta 350, 126–130 (2003).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
CrysAlisPRO version 39.46 (Rigaku Oxford Diffraction Ltd, 2018).
Sheldrick, G. M. SHELXT – integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45, 849–854 (2012).
Persistence of Vision (TM) Raytracer (Persistence of Vision Pty. Ltd, 2004).
Day, B. M. et al. Rare‐earth cyclobutadienyl sandwich complexes: synthesis, structure and dynamic magnetic properties. Chem. Eur. J. 24, 16779–16782 (2018).
Jolivet, J. P., Thomas, Y., Taravel, B., Lorenzelli, V. & Busca, G. Infrared spectra of cerium and thorium pentacarbonate complexes. J. Mol. Struc. 79, 403–408 (1982).
Karyakin, A. V. & Volynets, M. P. Infrared spectra of thorium-carbonate complex salts. J. Struct. Chem. 3, 689–690 (1962).
Clavier, N. et al. X-ray diffraction and μ-Raman investigation of the monoclinic-orthorhombic phase transition in Th1-xUx(C2O4)2·2H2O solid solutions. Inorg. Chem. 49, 1921–1931 (2010).
Kot, W. K., Shalimoff, G. V., Edelstein, N. M. Edelman, M. A. & Lappert, M. F. [Th(III)[η5-C5H3(SiMe3)2]3], an actinide compound with a 6d1 ground state. J. Am. Chem. Soc. 110, 986–987 (1988).
Blake, P. C. et al. Synthesis, properties and structures of the tris(cyclopentadienyl)thorium(III) complexes [Th{η5-C5H3(SiMe2R)2-1,3}3] (R=Me or tBu). J. Organomet. Chem. 636, 124–129 (2001).
Siladke, N. A. et al. Actinide metallocene hydride chemistry: C–H activation in tetramethylcyclopentadienyl ligands to form [μ-η5-C5Me3H(CH2)-κC]2– tuck-over ligands in a tetrathorium octahydride complex. Organometallics 32, 6522–6531 (2013).
Wedal, J. C., Bekoe, S., Ziller, J. W., Furche, F. & Evans, W. J. In search of tris(trimethylsilylcyclopentadienyl) thorium. Dalton Trans. 48, 16633–16640 (2019).
Alessi, A., Agnello, S., Buscarino, G., Pan, Y. & Mashkovtsev, R. I. in Applications of EPR in Radiation Research (eds Lund, A., Shiotani, M.) 255–295 (Springer International Publishing, 2014).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Küchle, W., Dolg, M., Stoll, H. & Preuss, H. J. Energy‐adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 100, 7535–7542 (1994).
Cao, X. & Dolg, M. Segmented contraction scheme for small-core actinide pseudopotential basis sets. J. Mol. Struct. THEOCHEM 673, 203–209 (2004).
Cao, X., Dolg, M. & Stoll, H. J. Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J. Chem. Phys. 118, 487–496 (2003).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Becke, A. D. & Johnson, E. R. A density-functional model of the dispersion interaction. J. Chem. Phys. 123, 154101 (2005).
Johnson, E. R. & Becke, A. D. A post-Hartree–Fock model of intermolecular interactions. J. Chem. Phys. 123, 024101 (2005).
Johnson, E. R. & Becke, A. D. A post-Hartree-Fock model of intermolecular interactions: Inclusion of higher-order corrections. J. Chem. Phys. 124, 174104 (2006)
Keith, T. A. AIMAll 19.10.12 (TK Gristmill Software, 2019).
Te Velde, G. et al. Chemistry with ADF. J. Comp. Chem. 22, 931–967 (2001).
ADF 2012.1 (SCM, Theoretical Chemistry, Vrije Universiteit, 2012).
Tsipis, A. C., Kefalidis, C. E. & Tsipis, C. A. The role of the 5f orbitals in bonding, aromaticity, and reactivity of planar isocyclic and heterocyclic uranium clusters. J. Am. Chem. Soc. 130, 9144–9155 (2008).
Bursten, B. E. & Ozin, G. A. Xα-SW calculations for naked actinide dimers: existence of ϕ bonds between metal atoms. Inorg. Chem. 23, 2910–2911 (1984).
Pepper, M. & Bursten, B. E. Ab initio studies of the electronic structure of the diuranium molecule. J. Am. Chem. Soc. 112, 7803–7804 (1990).
Cayton, R. H., Novo-Gradac, K. J. & Bursten, B. E. Metal-metal bonds involving the f elements. 4. Molecular orbital studies of metal-metal and metal-ligand interactions in dinuclear uranium(V) systems. Inorg. Chem. 30, 2265–2272 (1991).
Gagliardi, L. & Roos, B. O. Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond. Nature 433, 848–851 (2005).
Gagliardi, L., Pyykkö, P. & Roos, B. O. A very short uranium–uranium bond: the predicted metastable U22+. Phys. Chem. Chem. Phys. 7, 2415–2417 (2005).
Straka, M. & Pyykkö, P. Linear HThThH: a candidate for a Th−Th triple bond. J. Am. Chem. Soc. 127, 13090–13091 (2005).
Roos, B. O. & Gagliardi, L. Quantum chemistry predicts multiply bonded diuranium compounds to be stable. Inorg. Chem. 45, 803–807 (2006).
Cavigliasso, G. & Kaltsoyannis, N. Metal–metal bonding in molecular actinide compounds: electronic structure of [M2X8]2− (M = U, Np, Pu; X = Cl, Br, I) complexes and comparison with d-block analogues. Dalton Trans. 5476–5483 (2006).
La Macchia, G., Brynda, M. & Gagliardi, L. Quantum chemical calculations predict the diphenyl diuranium compound [PhUUPh] to have a stable 1Ag ground state. Angew. Chem. Int. Ed. 45, 6210–6213 (2006).
Roos, B. O., Malmqvist, P.-Å. & Gagliardi, L. Exploring the actinide−actinide bond: theoretical studies of the chemical bond in Ac2, Th2, Pa2, and U2. J. Am. Chem. Soc. 128, 17000–17006 (2006).
Roos, B. O., Borin, A. C. & Gagliardi, L. Reaching the maximum multiplicity of the covalent chemical bond. Angew. Chem. Int. Ed. 46, 1469–1472 (2007).
Wu, X. & Lu, X. Dimetalloendofullerene U2@C60 has a U−U multiple bond consisting of sixfold one-electron-two-center bonds. J. Am. Chem. Soc. 129, 2171–2177 (2007).
Cavigliasso, G. & Kaltsoyannis, N. Energy decomposition analysis of metal−metal bonding in [M2X8]2- (X = Cl, Br) complexes of 5f (U, Np, Pu), 5d (W, Re, Os), and 4d (Mo, Tc, Ru) elements. Inorg. Chem. 46, 3557–3565 (2007).
Raab, J., Lindh, R. H., Wang, X., Andrews, L. & Gagliardi, L. A combined experimental and theoretical study of uranium polyhydrides with new evidence for the large complex UH4(H2)6. J. Phys. Chem. A 111, 6383–6387 (2007).
Zhou, J., Sonnenberg, J. L. & Schlegel, H. B. Theoretical Studies of AnII2(C8H8)2 (An = Th, Pa, U, and Np) complexes: the search for double-stuffed actinide metallocenes. Inorg. Chem. 49, 6545–6551 (2010).
Manni, G. L. et al. Assessing metal–metal multiple bonds in Cr-Cr, Mo-Mo, and W-W Compounds and a hypothetical U-U compound: a quantum chemical study comparing DFT and multireference methods. Chem. Eur. J. 18, 1737–1749 (2012).
Dai, X. et al. Energetics and electronic properties of a neutral diuranium molecule encapsulated in C90 fullerene. Procedia Chem. 7, 528–533 (2012).
Penchoff, D. A. & Bursten, B. E. Metal–metal bonding in the actinide elements: onceptual synthesis of a pure two-electron U–U fδ single bond in a constrained geometry of U2(OH)10. Inorg. Chim. Acta 424, 267–273 (2015).
Su, D.-M., Zheng, X.-J., Schreckenbach, G. & Pan, Q.-J. Highly diverse bonding between two U3+ ions when ligated by a flexible polypyrrolic macrocycle. Organometallics 34, 5225–5232 (2015).
Wang, C.-Z. et al. Actinide (An = Th–Pu) dimetallocenes: promising candidates for metal–metal multiple bonds. Dalton Trans. 44, 17045–17053 (2015).
Foroutan-Nejad, C. et al. Bonding in U2@C80: cage-driven metal–metal bonds in di-uranium fullerenes. Phys. Chem. Chem. Phys. 17, 24182–24192 (2015).
Qu, N., Su, D.-M., Wu, Q.-Y., Shi, W.-Q. & Pan, Q.-J. Metal-metal multiple bond in low-valent diuranium porphyrazines and its correlation with metal oxidation state: a relativistic DFT study. Comp. Theo. Chem. 1108, 29-39 (2017).
Hu, H.-S. & Kaltsoyannis, N. The shortest Th–Th distance from a new type of quadruple bond. Phys. Chem. Chem. Phys. 19, 5070–5076 (2017).
Ge, X., Dai, X., Zhou, H., Yang, Z. & Zhou, R. Stabilization of open-shell single bonds within endohedral metallofullerene. Inorg. Chem. 59, 3606–3618 (2020).
Jaroš, A., Foroutan-Nejad, C. & Straka, M. From π bonds without σ bonds to the longest metal–metal bond ever: a survey on actinide–actinide bonding in fullerenes. Inorg. Chem. 59, 12608–12615 (2020).
Acknowledgements
We gratefully acknowledge funding and support from the UK Engineering and Physical Sciences Research Council (grants EP/K024000/1, EP/M027015/1, EP/P001386/1, EP/S033181/1 and EP/T011289/1), Natural Environment Research Council (grant NE/R011230/1) European Research Council (grant CoG612724), Royal Society (grant UF110005), Deutsche Forschungsgemeinschaft (SL104/10-1), the Landesgraduiertenförderung of the State of Baden-Württemberg and The University of Manchester (including computational resources and associated support services of the Computational Shared Facility). We also thank M. Jennings (Micro Analytical Laboratory, University of Manchester) for performing elemental microanalyses. S.T.L. thanks the Alexander von Humboldt Foundation for a Friedrich Wilhelm Bessel Research Award.
Author information
Authors and Affiliations
Contributions
J.T.B. prepared and characterized the complexes. J.A.S., D.H. and J.v.S. recorded and interpreted the magnetic and EPR data. A.W.W. and L.N. collected and analysed the solid-state UV–Vis data. A.J.W. collected, solved and refined all of the crystallographic data. N.K. performed and analysed the calculations. S.T.L. assisted with data analysis and directed the research. J.T.B., N.K. and S.T.L. wrote the manuscript with input from all of the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Pekka Pyykkö and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Spectroscopic and structural data for 3.
a, 1H NMR spectrum in C6D6 of a crude reaction mixture producing 3 showing unreacted 1 and the {C4(SiMe3)4} by-product. b, Polymeric structure of 3 at 150 K with 30% probability ellipsoids. H-atoms and disorder components omitted for clarity. Key: Th, green; K, dark blue; Cl, violet-red; O, red; C, grey. c, ATR-IR spectrum of 3 prepared in benzene. d, ATR-IR spectrum of 3 prepared in D6-benzene. e, 1H NMR spectrum of 3 in C6D6 after treatment with CCl4. Resonances at 2.15 and ~7.00-7.40 ppm correspond to residual toluene. f, 13C{1H} NMR spectrum of 3 in C6D6 after treatment with CCl4.
Extended Data Fig. 2 The two principal, colour-determining absorptions for 3".
Both transitions originate from the HOMO (MO 199) orbital. Hydrogen atoms are omitted for clarity.
Extended Data Fig. 3 Solid-state UV–Vis spectra of 3.
a, comparison of experimental spectrum (black) to the TD-DFT predicted spectrum presented as oscillator strengths (vertical red lines). b, comparison of the experimental spectrum before (black) and after (red) being allowed to oxidise in air.
Extended Data Fig. 4 NICSzz of 3" evaluated at intervals of R = 0.1 Å.
R is the perpendicular distance from the centre of the Th3 ring and the plane of the Cl ligands is at R = ±1.8 Å.
Extended Data Fig. 5 Vibrations of 3" with Th-Th character.
a, at 70.8 cm−1. b, at 71.0 cm−1. c, at 77.2 cm−1. d, at 107.4 cm−1. H-atoms are omitted for clarity.
Extended Data Fig. 6 Magnetic and EPR Data for 3.
a, Raw magnetic moment recorded by variable-temperature SQUID magnetometry on a flame sealed borosilicate tube containing a sample of 3 in a 1T field with the data for a blank tube subtracted. b, Molar paramagnetic susceptibility χ per Th3 unit recorded by variable-temperature SQUID magnetometry (black symbols). Expected curves for a d1 (S = ½) system with g = 2 (blue,) for a non-correlated d1-d1 (2 × S = ½) system, also with g = 2 (red), and for a triplet d1-d1 (S = 1) system (green). c, X-Band EPR spectra recorded on powdered 3 in a flame-sealed quartz tube at different temperatures as indicated. d, Room temperature X-Band EPR spectra recorded on a loose powder of 3 in a flame-sealed quartz tube. Simulation of the EPR spectrum assuming two species in a 20:80 ratio with principal g-tensor values of gx1 = 1.9965, gy1 = 2.0036, gz1 = 2.010, and gx2 = 1.9375, gy2 = 1.9762, gz2 = 1.9747, respectively.
Extended Data Fig. 7 NMR spectroscopic data for the treatment of thorocene and 2 with CO2.
a, 1H NMR spectrum in C6D6 of thorocene after treatment with excess CO2, recorded after 2 h. b, 1H NMR spectrum in C6D6 of 2 after treatment with excess CO2, recorded after 2 h.
Extended Data Fig. 8 NMR and IR spectroscopic data for the reaction of 3 with CO2.
a, 1H NMR spectrum in C6D6 of the mother liquor after 3 is treated with excess CO2. Trace toluene resonances are from the preparation of 3. b, 13C{1H} NMR spectrum in C6D6 of the mother liquor after 3 is treated with excess CO2. The use of an excess of CO2 is reflected by the small resonance for CO2. c, IR spectrum of the product of the reaction of 3 with excess CO2 with key absorptions at 1540 and 1371 cm−1 indicative of carbonate and not oxalate formation.
Extended Data Fig. 9 NMR spectroscopic data for the reaction of 3 with C8H8.
a, 1H NMR spectrum in C6D6 of the mother liquor after sonicating and heating 3 with C8H8. b, 13C{1H} NMR spectrum in C6D6 of the mother liquor after sonicating and heating 3 with C8H8.
Supplementary information
Supplementary Table 1
Final single point energy and coordinates for geometry optimized 3'.
Supplementary Table 2
Final single point energy and coordinates for geometry optimized 3"
Supplementary Table 3
Final single point energy and coordinates for geometry optimized [{Th(η8-C8H8)(μ3-Cl)2}3K2]2+.
Supplementary Table 4
Final single point energy and coordinates for geometry optimized [{Th(η8-C8H8)(μ3-Cl)2}3].
Supplementary Table 5
Final single point energy and coordinates for geometry optimized [{Th(η8-C8H8)(μ3-Cl)2}3H]–.
Supplementary Table 6
Final single point energy and coordinates for geometry optimized [{Th(η8-C8H8)(μ3-Cl)2}3K2H]+.
Supplementary Table 7
Final single point energy and coordinates for geometry optimized [{Th(η8-C8H8)(μ3-Cl)2}3K2H].
Rights and permissions
About this article
Cite this article
Boronski, J.T., Seed, J.A., Hunger, D. et al. A crystalline tri-thorium cluster with σ-aromatic metal–metal bonding. Nature 598, 72–75 (2021). https://doi.org/10.1038/s41586-021-03888-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03888-3
This article is cited by
-
Thorium(iv)–antimony complexes exhibiting single, double, and triple polar covalent metal–metal bonds
Nature Chemistry (2024)
-
Actinide-lanthanide single electron metal-metal bond formed in mixed-valence di-metallofullerenes
Nature Communications (2023)
-
φ-Aromaticity in prismatic {Bi6}-based clusters
Nature Chemistry (2023)
-
[{Th(C8H8)Cl2}3]2− is stable but not aromatic
Nature (2022)
-
Ring contraction of metallacyclobutadiene to metallacyclopropene driven by π- and σ-aromaticity relay
Nature Synthesis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.