Abstract
Complexes of iron in high oxidation states are captivating research subjects due to their pivotal role as active intermediates in numerous catalytic processes. Structural and spectroscopic studies of well-defined model complexes often provide evidence of these intermediates. In addition to the fundamental molecular and electronic structure insights gained by these complexes, their reactivity also affects our understanding of catalytic reaction mechanisms for small molecule and bond-activation chemistry. Here, we report the synthesis, structural and spectroscopic characterization of a stable, octahedral Fe(VI) nitrido complex and an authenticated, unique Fe(VII) species, prepared by one-electron oxidation. The super-oxidized Fe(VII) nitride rearranges to an Fe(V) imide through an intramolecular amination mechanism and ligand exchange, which is characterized spectroscopically and computationally. This enables combined reactivity and stability studies on a single molecular system of a rare high-valent complex redox pair. Quantum chemical calculations complement the spectroscopic parameters and provide evidence for a diamagnetic (S = 0) d 2 Fe(VI) and a genuine S = 1/2, d 1 Fe(VII) configuration of these super-oxidized nitrido complexes.

Similar content being viewed by others
Main
Iron—the most abundant transition element—and its high oxidation state chemistry are of fundamental importance in various (bio)catalysts1,2,3,4. In this regard, the electronic structure and reactive nature of highly oxidized iron complexes are relevant for a general understanding of such catalytic processes5,6,7. In stark contrast to the notable stability of the permanganate ion [MnO4]–, the neighbouring ferrate(VII) anion [FeO4]–, generated by photolysis of the dioxoiron peroxide [(η2-O2)FeO2]–, is believed to be stable in helium matrices only at 4 K (refs. 8,9). With the exception of the shelf-stable, commercially available tetraoxoferrate(VI) dianion—which splits water into dioxygen10,11 and is also used for environmentally friendly water treatment12—only a handful of high-oxidation-state iron compounds are known. The hitherto accessible molecular compounds have been terminal Fe(IV,V) nitrido13,14,15,16,17,18,19, imido20,21,22,23 and oxo24,25,26,27,28,29 complexes, with their diverse reactivity attributed to the axial metal–ligand multiple bonds30,31,32. By contrast, only two Fe(VI) complexes have been reported before. These are a spectroscopically observed Fe(VI) nitride33, and a structurally authenticated, tetrahedral Fe(VI) bis(imide)34. Herein, we report the synthesis, isolation and full characterization of an octahedral Fe(VI) nitride (1). One-electron oxidation of 1 yields the unique Fe(VII) intermediate 2, which is unambiguously spectroscopically characterized and rearranges to an Fe(V) imide product (3) (Fig. 1a). The calculated energy profile for the latter transformation provides further credence to a heptavalent iron complex (see below). Single-crystal X-ray diffraction (SC-XRD) studies of 1 and 3 revealed six-coordinate Fe centres in both compounds. The formation of the intramolecular amination product 3 emphasizes the high reactivity of the super-oxidized Fe(VII) intermediate 2.
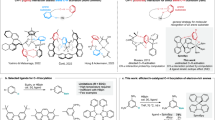
a, Oxidation of the Fe(VI) nitride (1) leads to a reactive Fe(VII) nitride (2) (MF6 with M = Mo, Re), which readily rearranges intramolecularly via C–C and C–H bond activation chemistry to a high-valent, unusual Fe(V) imide (3). b, Molecular structure of dication 1 in crystals of [(TIMMNMes)FeVI(N)(F)](PF6)2·CH2Cl2, depicting the coordination environment of the central hexavalent Fe ion, as well as the short Fe≡N bond length of 1.518(3) Å. Thermal ellipsoids are shown at 50% probability; counterions, hydrogen atoms and co-crystalized solvent molecules are omitted for clarity. c, Solid-state zero- and applied-field 57Fe Mössbauer spectra of 1 at 77 K (top) and 1.7 K (bottom). The coloured lines represent the best global fit for the experimental data (black circles) with a remarkably negative isomer shift of −0.60 mm s−1. d, Experimental Fe K-edge X-ray absorption spectrum of 1, measured in Kβ1,3-HERFD mode, and the corresponding TDDFT-calculated spectrum (see Supplementary Information for details).
Results and discussion
Synthesis and characterization of the hexavalent iron nitride
Oxidation of the [(TIMMNMes)FeV(N)]2+ complex I (TIMMNMes = tris-[(3-mesityl-imidazol-2-ylidene)methyl]amine)17 with silver difluoride (AgIIF2) provides quantitative access to the hexavalent nitrido complex [(TIMMNMes)FeVI(N)(F)]2+ (1). The molecular structure of 1 (Fig. 1b), determined by SC-XRD analysis, reveals a six-coordinate iron centre best described as a Cs-symmetric, distorted octahedron due to the additional, equatorially coordinated fluorido ligand. The iron centre is situated above the tris-carbene plane with an offset (doop) of 0.419(3) Å and a short Fe≡N bond length of 1.518(3) Å. The average Fe−CNHC distance is 1.976(4) Å with the shortest Fe−C bond length of 1.967(4) Å trans to the fluorido ligand, whereas the Fe−Namine distance amounts to 2.351(3) Å.
Multinuclear 1H, 13C, 15N and 19F nuclear magnetic resonance (NMR) experiments also confirm that 1 is diamagnetic and Cs-symmetric, unlike its trigonal-symmetric Fe(IV) and Fe(V) precursors and related imido complexes of the TIMMNMes ligand17,20. In solution, the fluorido ligand remains bound to the iron centre, as evidenced by an unusual 19F NMR chemical shift of −310 ppm (versus CFCl3; Supplementary Fig. 3), and couplings to the 13C and 15N nuclei in the respective NMR spectra. The zero-field 57Fe Mössbauer spectrum of 1 (Fig. 1c), recorded in the solid state at 77 K, shows a sharp quadrupole doublet with a remarkably negative isomer shift of −0.60 mm s−1 and a quadrupole splitting (ΔEQ) of 4.16 mm s−1, which is indicative of an Fe(VI) ion with a d 2, S = 0 electronic ground state (1.7 K, 7 T: δ = –0.58 mm s−1, ΔEQ, = +4.16 mm s–1, η = 0.54). Still, the negative isomer shift—indicative of a highly oxidized Fe ion—is much more negative than observed for the only other reported Fe(VI) complexes, namely, [(Me3cy-ac)FeVI(N)](PF6)2 (cy-ac = 1,4,8,11-tetraazacyclotetradecane-1-acetate) (δ = −0.29 mm s−1, Fe−N distance (EXAFS) = 1.57(2) Å)33 and [H2B(MesIm)2FeVI(=NMes)2]BF4 (H2B(MesIm)2 = dihydro-bis[(1-mesityl)imidazol-2-ylidene]borato) (δ = −0.48 mm s−1, Fe−N distance = 1.634(3) Å)34. The markedly more negative isomer shift observed in hexavalent 1, when compared with these other Fe(VI) complexes, can be attributed to the shorter, more covalent Fe−N bond in 1. The effect of the ligand charge, first-shell ligand atoms, and coordination number require further in-depth analysis, as reported elsewhere35. X-ray absorption spectroscopy (XAS) measurements were conducted to elucidate further the electronic structure of hexavalent 1 and its related tetra- and pentavalent Fe(IV,V) nitrido complexes. The Fe Kβ1,3-HERFD XAS spectrum of [(TIMMNMes)FeVI(N)(F)]2+ (1) (Fig. 1d) shows a slightly asymmetric pre-edge peak at 7,114.9 eV and a minor feature on the onset of the rising edge at 7,119.2 eV. The inflection point at 7,126.9 eV of the rising edge follows the oxidation state trend of [FeIV,V(N)]1+/2+ (I′, I), with the energies of 7,123.9 and 7,125.4 eV being consistent with a +VI oxidation state in 1 (Supplementary Fig. 17). These values are considerably higher than for other reported Fe(VI) complexes33,34 due to the 0.05 Å shorter Fe−N bond length in 1 compared with the experimentally determined EXAFS Fe−N data in [(Me3cy-ac)FeVI(N)](PF6)2 (ref. 33). This trend is reflected within the more negative 57Fe Mössbauer isomer shift values of this Fe nitride series. The Cs symmetry of the complex stabilizes the 3d(xy) and 3d(x²–y²) orbitals relative to the parent Oh levels, causing the two d electrons to be paired in 3d(xy), thus, leading to the electronic configuration a″(xy)2, a′(xz)0, a″(yz)0, a′(x²–y²)0, a′(z²)0, as supported by quantum chemical calculations (Supplementary Fig. 48)36,37,38,39.
Formation of the heptavalent iron nitride
Unexpectedly, the particularly stable Fe(VI) complex 1 can be oxidized even further using powerful oxidizing agents, such as the metal hexafluorides (MF6 with M = Re, Mo) or salts containing the XeF+ cation (Supplementary Fig. 10). Treatment of 1 with either one of these reagents forms the one-electron-oxidized Fe(VII) nitride [(TIMMNMes)FeVII(N)(F)]3+ (2). This super-oxidized complex is highly reactive above −50 °C and rearranges (Fig. 2b,e) via an intramolecular amination mechanism40,41 to the cyclic Fe(V) imido complex [(TIMMNMes*)FeV(=N*)(NCMe)]3+ (3) (Fig. 2c,f). The freeze–quench 57Fe Mössbauer and X-band electron paramagnetic resonance (EPR) studies (Fig. 2), combined with XAS techniques (Supplementary Fig. 14), enabled the identification of 2. The initial zero-field 57Fe Mössbauer spectrum (Fig. 2a) reveals 2 as the main component (64%) with an extraordinary negative isomer shift of −0.72 mm s−1 and ΔEQ of 3.30 mm s−1. With the exception of ferrates (δ ≈ −0.80 mm s−1 at 78 K)42, the isomer shift in 2 represents the most negative value reported for any other known molecular iron coordination complex (see Supplementary Information for details), suggesting that the iron centre in 2 is the most strongly oxidized one. A linear correlation plot of the 57Fe Mössbauer isomer shift versus the iron oxidation state further supports an Fe(VII) physical oxidation state (Fig. 3a). A similar analysis was reported for Fe(VI)33,34. The remarkably linear relationship between the 57Fe Mössbauer isomer shift and the oxidation state is surprising, considering the complexes’ different structures. While I′ is trigonal four-coordinate, I is five-coordinate, and 1 and 2 are tetragonal six-coordinated with an extra fluorido ligand bound in the equatorial plane. The linear correlation reported between the d-electron count and the calculated electron density at the Fe nucleus35 indicates that the contraction of s orbitals—while the effective nuclear charge increases with the oxidation state—seems to be the dominant factor, overriding structural discrepancies across the Fe(IV–VII) series. The EPR spectroscopic data of 2 (Fig. 2d) reveal a doublet (S = 1/2) ground state with an anisotropic, rhombic g-tensor with g1 = 2.058, g2 = 1.998, g3 = 1.908, and a super-hyperfine coupling to the nitrido nitrogen with A3 = 6.8 mT (14N, 99.63%, I = 1), which is indicative of a metal-centred spin. This characteristic three-line super-hyperfine-interaction with one coupled 14N nucleus vanishes upon thermally induced cyclization of 2 (Fig. 2d–f), and further matches the spectroscopic fingerprint (see below) of an independently synthesized sample of 3 (Supplementary Information). Owing to its diamagnetic (d 2, S = 0) ground state, unreacted 1 is EPR silent and, therefore, not seen in the spectrum of 2 (Fig. 2d).
a, A mixture of Fe(VI)N (1) and Fe(VII)N (2) trapped in frozen solution and studied by zero-field 57Fe Mössbauer spectroscopy. While the Fe(VI) nitride possesses an isomer shift of –0.60 mm s−1, the Fe(VII) nitride reveals an exceptional negative isomer shift of −0.72 mm s−1 within the Fe nitride series. b, Rearrangement of the Fe(VII) nitride (2) to yield the Fe(V) imide (3). c, The zero-field 57Fe Mössbauer spectrum after complete conversion of 2 to 3, with a less negative isomer shift of −0.16 mm s−1, typical for an Fe(V) imide. d–f, Formation, rearrangement and complete transformation of 2 to 3, followed by X-band EPR spectroscopy. The Fe(VII) possesses an S = 1/2 signal (g1 = 2.058, g2 = 1.998, g3 = 1.908) with a characteristic three-line nitrogen hyperfine coupling (A3 = 6.8 mT) that vanishes following the rearrangement. 57Fe Mössbauer spectra a–c were recorded at 77 K and EPR spectra d–f at 15 K. Black circles (top) and lines (bottom) represent the experimental data, whereas the coloured lines represent the best fit obtained.
a, Correlation diagram of the experimental (exp.) 57Fe Mössbauer isomer shift versus the Fe oxidation state of 1 and 2 compared with [(TIMMNMes) FeIV,V(N)]1+,2+ (I′, I). b, Correlation diagram of the exp. 57Fe Mössbauer isomer shift versus the calculated Bader atomic partial charge (quantum theory of atoms in molecules, Perdew–Burke-Ernzerhof functional) on Fe increasing within the I′–2 series. c, Overlay of XAS spectra of 1, deconvoluted 2 (see Supplementary Information for details) and [(TIMMNMes)FeIV,V(N)]1+,2+ (I′, I). The inflection points constantly shift to higher energies within the series (7,123.9 eV (I′), 7,125.4 eV (I), 7,126.9 eV (1) and 7,128.1 eV (2)), supporting the physical +VII oxidation state in 2. d, Pertinent natural orbitals of the metal–nitrido moiety in 2 according to CASSCF(15,15) calculations, lead configuration: c = 0.66 (compare wth Supplementary Fig. 53), revealing three covalent bonds with the nitrido ligand (yellow) and an unpaired electron (purple) in the d(xy) orbital (singly occupied molecular orbital), corroborating a metal-centred spin for an Fe(VII), d 1 S = 1/2. Mesityl groups are truncated with methyl groups for clarity, yet have been included in the calculations.
The deconvoluted XAS data of [(TIMMNMes)FeVII(N)(F)]3+ (2) (Fig. 3c and Supplementary Fig. 14), from the reaction mixture evaporated at −50 °C, reveal a similar overall spectrum compared with 1, but shifted to higher energies with an asymmetric pre-edge peak at 7,115.1 eV and a shoulder at 7,112.6 eV. Most importantly, the inflection point at 7,128.1 eV is 1.2 eV higher than for 1, thus, following the oxidation state trend and supporting the physical +VII oxidation state assignment of 2 (Fig. 3c). Quantum chemical calculations at various levels of theory show a delocalization of the unpaired electron across the iron centre and to a minor extent to the NHC ligands as well as to the mesityl groups, supporting the metal as the main locus of oxidation. CASSCF(15,15) computations of 2, which include the metal as well as the NHC- and mesityl substituents, identify three covalent bonds with the nitrido ligand and an unpaired electron in the d(xy) orbital (Fig. 3d; Supplementary Figs. 52 and 53; see Supplementary Fig. 48 for a comparison with 1). While the computed spin density at the iron atom is method-dependent, we find that including the polar acetonitrile solvent and the hexafluorophosphate anions in the calculations corroborates metal-centred character. Density functional theory calculations reproduce the experimental Mössbauer data and support an increasing accumulation of positive charge on the Fe ion within each oxidation step going from +IV to +V, +VI, and +VII (Fig. 3b; see also Supplementary Tables 10–14 and Supplementary Figs. 31–34).
Reactivity and mechanistic studies
When leaving frozen-matrix conditions, a rapid intramolecular rearrangement reaction occurs (Fig. 4a), thus, thwarting the isolation of heptavalent 2. Low-temperature crystallization attempts at −30 °C provided further mechanistic clarification, revealing that the Fe(V) imido complex [(TIMMNMes*)FeV(=N*)(NCMe)]3+ (3) (Fig. 4c) forms as the stable amination product. Following oxidization of 1 to 2, complex 3 forms through subsequent HF−MeCN ligand exchange under ambient conditions. Single-crystal X-ray diffraction analysis corroborates the Cs-symmetric nature of 3 and a retained six-coordinate Fe centre with a doop of 0.399(3) Å. Due to HF elimination43, the newly formed aryl-imido metallacycle possesses a typical20,23 FeV=Nimido bond length of 1.679(3) Å and an N−C bond length of 1.340(4) Å. Consequently, the aryl substituents of the ligand framework in 3 align parallel to each other in a graphene-type fashion with an intramolecular aryl−aryl distance of 3.343 Å (Supplementary Fig. 24). The average Fe−CNHC distance is 1.936(4) Å with a remarkably short Fe−C bond length of 1.855(4) Å trans to the acetonitrile ligand, and the Fe−Namine distance amounts to 2.172(3) Å. The zero-field 57Fe Mössbauer spectrum of 3 (Fig. 2c) is characteristic for a d 3, S = 1/2 electronic ground state of an Fe(V) imide20,34 with an isomer shift of −0.16 mms−1 and ΔEQ of 2.28 mms−1. The EPR spectroscopic data (Fig. 2f) confirm the low-spin (S = 1/2) state with an anisotropic g-tensor (g1 = 2.065, g2 = 2.000, g3 = 1.984). The overall reaction sequence is evidenced by quantum chemical calculations (Fig. 4a). An electrophilic attack of the nitrido ligand on the chelate’s mesityl group in 2 causes an initial N−C bond formation (Fig. 4a, complex a). The arenium ion rearranges by a 1,2-methyl shift (Fig. 4a, complex b) and a subsequent proton migration via the mesityl rings (Supplementary Fig. 27, complexes c and d) to afford the Fe(V) amido complex (Fig. 4a, complex e). The following elimination of HF is caused by the coordination of an incoming acetonitrile solvent molecule, which renders Fe(V) imido complex 3 thermodynamically stable. Notably, the migration of the methyl group, involving a 1,2-methyl shift followed by proton migration, has an electronic and steric origin. First, the 1,2-methyl shift allows for better stabilization of positive charge in the meta-position of the mesityl substituent (Fig. 4a, complexes a versus b). Second, as the Fe-nitrido nitrogen atom forms the metallacycle through the electrophilic attack and N–C bond formation, the methyl group probably clashes with the neighbouring aryl groups. The following 1,2-methyl shift and subsequent H+ transfer release the steric pressure, rearomatize the arene and allow the three aryl rings to align in a graphene-type π-stacking. At first, this reaction sequence yields a unique but unstable Fe(V) amido species (Fig. 4b, complex e), which, coincidentally, has been crystallographically characterized from HF (Supplementary Fig. 25). Ultimately, the stable Fe(V) imide (3) is formed via H–F elimination. Computing the corresponding reaction for hexavalent 1 confirms that the nitrido ligand is not sufficiently electrophilic for a reaction with the mesityl group, which renders the addition step considerably endergonic (see Supplementary Fig. 28).
a, Computed reaction profile (ZORA-TPSSh-D3(CPCM)/def2-TZVPP//ZORA-PBE-D3/def2-SVP). Proton transfer sequences c and d are omitted for clarity (see Supplementary Fig. 27). b, Molecular structure of intermediary Fe(V)amide 2e in crystals of [(TIMMNMes*)FeV(NH*)(F)](PF6)3, depicting the coordination environment of the Fe ion (dFe–Namido = 1.752(3) Å). c, Molecular structure of 3 in crystals of [(TIMMNMes*)FeV(=N*)(NCMe)](PF6)2(MoF6)·5 CH3CN depicting the coordination environment of the central pentavalent Fe ion with a typical Fe=Nimido bond length of 1.679(3) Å. All thermal ellipsoids for b and c are shown at 50% probability; counterions, hydrogen atoms and co-crystalized solvent molecules are omitted for clarity, except the amido proton in b and the acetonitrile hydrogens in c.
Together, these spectroscopic data corroborate the conversion of a high-valent Fe(VI) nitride (1) to a reactive, super-oxidized, heptavalent iron nitride (2) en route to an Fe(V) imide (3) via an intramolecular amination mechanism. This study, thus, establishes the full structural characterization of a molecular hexavalent Fe(VI) nitride and the spectroscopic identification of a genuine heptavalent Fe(VII) nitrido complex.
Methods
General considerations
All experiments were performed under a dry nitrogen atmosphere, using standard Schlenk techniques or an MBraun inert-gas glovebox, equipped with a –35 °C freezer. Solvents were purified using a two-column solid-state purification system (Glass Contour System) and stored over activated molecular sieves (3 Å) under an inert atmosphere for all halogenated, nitro- or nitrile-containing solvents (dichloromethane, chloroform, acetonitrile and nitromethane). Furthermore, potassium was added to alkanes, aromatic solvents and cyclic ethers (n-pentane, n-hexane, benzene, toluene and tetrahydrofuran). Sulfur dioxide (SO2) is a toxic gas (boiling point −10 °C) and was stored in a stainless-steel cylinder over CaH2. Anhydrous HF is a highly toxic and corrosive gas (boiling point = +19 °C), which was purified by distillation and stored in a stainless-steel cylinder. MoF6 and ReF6 (ABCR) are highly volatile liquids, corrosive and strongly oxidizing, and were also stored in stainless-steel cylinders. Reactions involving SO2, HF, MoF6 and ReF6 were performed in tetrafluoroethene-perfluoroalkoxyvinyl-copolymer (PFA) tubes connected to stainless-steel valves. Deuterated solvents were obtained, packaged under argon and stored over activated molecular sieves. The NMR spectra were recorded on a JEOL ECZ 400 S instrument operating at 399.79 MHz for 1H and 100.53 MHz for 13C. The solvent residual signals of incomplete deuterated solvent molecules were used as the internal reference for the 1H NMR spectra and the solvent signals for 13C NMR spectral data44. For 15N, 19F and 31P NMR data, chemical shifts were referenced by the Delta v.5.0.5 software provided by JEOL Ltd NMR. Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, b = broad. Coupling constants J are given in hertz. Zero-field 57Fe Mössbauer spectra were recorded on a WissEl Mössbauer spectrometer (MRG-500) at a temperature of 77 K in constant acceleration mode. 57Co/Rh was used as γ-radiation source (1.8 GBq). The temperature of the samples was controlled by a MBBC-HE0106 MÖSSBAUER He/N2 cryostat within an accuracy of ±0.3 K. Applied-field 57Fe Mössbauer spectra were recorded with alternating constant acceleration of the γ-source (lab-built spectrometer, MPI-CEC). The sample temperature was maintained constant in a cryogen-free, closed-cycle Mössbauer magnet cryostat from Cryogenic Ltd. The latter is a split-pair super-conducting magnet system for applied fields of up to 7 T. The temperature of the sample can be varied in the range 1.7 K to 300 K. Isomer shifts are reported relative to α-iron metal at 300 K. Electron paramagnetic resonance spectra were recorded under nitrogen atmosphere in air-tight J. Young quartz glass EPR tubes on a JEOL continuous wave spectrometer (JES-FA200) equipped with an X-band Gunn diode oscillator bridge, a cylindrical mode cavity, and a helium cryostat using the following parameters: microwave frequency = 8.959 GHz, modulation width = 1.0 mT, microwave power = 1.0 mW, modulation frequency = 100 kHz, and time constant = 0.1 s. Electron paramagnetic resonance line widths, W, are given in units of millitesla at the full-width at half-maximum. X-ray absorption spectroscopy in high-energy resolution fluorescence-detected mode (Kβ-HERFD) was performed at the European Synchrotron Radiation Facility (ESRF) beam line ID26 (6 GeV, 30 mA, 16-bunch mode) using a Si(311) liquid nitrogen cooled double crystal monochromator. The emission spectrometer was set to the maximum of Kβ1,3 line and the absorption spectrum was collected in continuous scan mode from 7,080 to 7,250 eV with integration set to give a 0.1 eV step size. Scans from 7,020 to 7,760 eV were collected for normalization. The final data were composed of an average of scans on 60–80 spots on the surface of a single sample. X-ray absorption spectroscopy in transmission mode was performed at the Stanford Synchrotron Radiation Lightsource beamline 9-3 (3 GeV, 500 mA, top-off mode) using a Si(220) liquid-nitrogen-cooled double crystal monochromator. All samples were measured at 10 K in a liquid helium flow cryostat. The XAS measurements were collected in step-scan mode from 6,830.8 to 7,095.8 in 5 eV steps, from 7,096.0 to 7,140.0 in 0.2 eV steps, and at constant steps in k space up to 7,988 eV. Electronic absorption spectra were recorded in solution on a Shimadzu double-beam UV-3600 UV/Vis/NIR spectrophotometer at room temperature. Electrochemical measurements were performed at room temperature under a dinitrogen atmosphere with an µAutolab Type III potentiostat using a rotating disk electrode with a glassy carbon or platinum tip (3 mm diameter) as working electrode and platinum wires as counter and pseudo-reference electrodes referenced to the Fc+/Fc couple. Elemental analyses were obtained using Euro EA 3000 (Euro Vector) and EA 1108 (Carlo-Erba) elemental analysers at the Friedrich-Alexander-Universität Erlangen-Nürnberg. See the Supplementary Information for in-depth details on the parameters of each spectroscopic set-up.
Starting materials
Commercially available starting materials were purchased from commercial suppliers (Acros Organics, Alfa Aesar, Sigma-Aldrich, Merck, TCI, VWR) and were used without further purification. 1-Mesitylimidazole, tris-(chloromethyl)amine, tris-[(3-mesityl-imidazol-2-ylidene)-methyl]amine (TIMMNMes) and [(TIMMNMes)FeIV,V(N)]1+,2+ (I′/I) were synthesized as reported in the literature17,45,46,47.
Synthetic procedures
[(TIMMNMes)FeVI(N)(F)](PF6)2 (1)
Excess (3–4 equiv.) silver difluoride (AgF2) was added, under the exclusion of light, to a solution of [(TIMMNMes)FeV(N)](PF6)2 (I) (400 mg, 0.41 mmol, 1.0 equiv.) in dichloromethane (10 ml). The reaction mixture was vigorously stirred for 1 h at room temperature, whereupon a gradual colour change from intensively yellow–orange to lime-green occurred. This colour change is accompanied by the formation of a green precipitate of the crude [(TIMMNMes)FeVI(N)(F)](PF6)2 complex 1. The resulting dark green solid was filtered off, washed with dichloromethane (4 × 2 ml), redissolved in acetonitrile, and extracted from the remaining AgF2 excess by filtration. Subsequent drying in vacuo yielded 350 mg (85%) of lime-green powdery complex 1 used for spectroscopic analysis. Single-crystals suitable for X-ray diffraction analysis were obtained by slow diffusion of diethyl ether into an acetonitrile/dichloromethane mixture at –35 °C overnight.
1H NMR (400 MHz, CD3NO2, +23 °C) δ 7.76 (s, 3 H, Im-H), 7.56 (s, 3 H, Im-H), 7.01 (s, 2 H, Ar-H), 6.99 (s, 2 H, Ar-H), 6.67 (s, 2 H, Ar-H), 5.86 (s, 2 H, N–CH2), 5.70 (d, |2J| = 11.9 Hz, 2 H, N–CH2), 5.38 (d, |2J| = 11.9 Hz, 2 H, N–CH2), 2.34 (s, 6 H, CH3), 2.32 (s, 6 H, CH3), 2.23 (s, 3 H, CH3), 1.97 (s, 6 H, CH3), 1.40 (s, 6 H, CH3). 13C NMR (101 MHz, CD3CN, +23 °C δ 167.82 (2C, Fe–CN2), 157.26 (d, 2JC,F = 29.0 Hz, 1 C, Fe–CtransN2), 141.85, 141.64, 136.61, 136.48, 135.68, 134.86 and 133.02 (12C, Ar-C), 133.67, 130.82, 130.38, 130.18, 129.37, 124.17, and 123.74 (12C, Ar-CH and Im-CH), 73.36 (1C, N–CH2), 72.32 (2C, N–CH2), 21.11, 21.06, 19.08, 18.61 and 17.52 (9C, CH3). 15N NMR (41 MHz, CD3CN, +23 °C) δ 1057.5 (d, 2JN,F = 22 Hz, Fe≡N). 19F NMR (376 MHz, CD3CN, +23 °C) δ –72.67 (d, 1JF,P = 706.6 Hz, (PF6–)), –309.54 (s, Fe–F). 31P NMR (162 MHz, CD3CN, +23 °C) δ –141.55 (septet, 1JP,F = 706.6 Hz, (PF6–)). Elemental analysis (calc., found for C39H45FeN8P2F13·1CH2Cl2): C (44.67, 44.74), H (4.40, 4.28), N (10.42, 10.45).
[(TIMMNMes)FeVII(N)(F)](PF6)2(PF6/MF6), M = Mo, Re (2)
The following procedures have been applied for freeze–quench spectroscopic experiments (57Fe Mössbauer, EPR and X-ray absorption spectroscopy). Route 1: excess (3–5 equiv.) XeF2 and sodium hexafluorophosphate (7 mg, 0.04 mmol, 1.0 equiv.) were added to a pre-cooled solution (−30 °C) of [(TIMMNMes)FeVI(N)(F)](PF6)2 (1) (40 mg, 0.04 mmol, 1.0 equiv.) in acetonitrile (1 ml). The reaction mixture was vigorously stirred for a few minutes at −30 °C, whereupon a gradual colour change from intensively lime-green to orange–red occurred. This mixture was immediately frozen and analysed spectroscopically. Note: a similar observation was made using XeF(SbF6) in chlorofluoromethane at −60 °C. Herein, an immediate colour change from lime-green to red occurred. Route 2: [(TIMMNMes)FeVI(N)(F)](PF6)2 (1) (20 mg, 0.02 mmol, 1.0 equiv) was placed inside a PFA tube (8 mm outer diameter) equipped with a stainless-steel valve and connected to a stainless-steel vacuum line. The PFA tube was cooled to −196 °C with liquid nitrogen, and ∼1.0 ml of SO2 (which is highly oxidation stable) was condensed onto 1. The tube was placed in a −10 °C ethanol cooling bath to liquefy SO2. Afterwards, the PFA tube was again cooled to −196 °C, and an excess of MF6 (20–30 mg) was condensed into the reaction vessel. The PFA tube was then placed again in a cold ethanol bath. For ReF6, a temperature of −60 °C was already sufficient to observe a colour change to orange–red within minutes, whereas, in the case of MoF6, the reaction temperature was carefully raised successively to −10 °C. After approximately 10 min, all volatiles were removed as much as possible in vacuum in the cold (−60 °C, 10–3 mbar); however, at the end, warming to room temperature for 1–2 min was needed to ensure complete removal of all volatiles to give an orange–red solid. After removal of all volatiles, the PFA tubes were flame-sealed in vacuum and the samples were placed in a cryoshipper (liquid nitrogen temperature) and sent from Berlin to Erlangen/Mülheim for further spectroscopic analysis. Note: the first route is preferred for monitoring the formation, rearrangement and conversion of 2 by freeze–quench studies with 57Fe Mössbauer and X-band EPR spectroscopy, whereas the second route enables handling/removal of reactants at lower temperatures (−60 °C, 10–3 mbar) and, therefore, allows for a solid-state 57Fe Mössbauer and X-ray absorption spectroscopic analysis. Note: the intramolecular rearrangement of 2 to 3 occurred independently from the solvent used in each route, as a fraction of acetonitrile remains within 1 following extraction of [(TIMMNMes)FeVI(N)(F)](PF6)2 from AgF2 (see above).
[(TIMMNMes*)FeV(=N*)(NCMe)](PF6)2(MF6), M = Mo, Re (3)
Complex 3 was prepared in analogy to the freeze–quench experiments following the synthesis of 2. Accordingly, [(TIMMNMes)FeVI(N)(F)](PF6)2 (1) (20 mg, 0.02 mmol, 1.0 equiv.) was placed inside of a PFA tube equipped with a stainless-steel valve and connected to a stainless-steel vacuum line, cooled to −196 °C with liquid nitrogen, and ∼1.0 ml of SO2 was condensed onto 1. The tube was placed in a −10 °C ethanol cooling bath to liquefy SO2. The PFA tube was then cooled again to −196 °C and excess MF6 (20–30 mg) was condensed onto the reactant. The PFA tube was then placed again in a cold ethanol bath and stirred for 15 min. Removal of all volatiles at −10 °C in vacuo produces an orange–brown, room-temperature-stable solid in quantitative yield, used for spectroscopic analysis. Single-crystals suitable for X-ray diffraction analysis of [(TIMMNMes*)FeV(=N*)(NCMe)](PF6)2(MF6), M=Mo, Re (3) can be obtained from acetonitrile/diethyl ether or acetonitrile/toluene mixtures. Elemental analysis (calc., found for C41H47FeN9P2MoF18·1C7H8·1.5CH3CN): C (44.72, 44.65), H (4.38, 4.51), N (10.69, 10.56). See the main text and Supplementary Information for additional in-depth spectroscopic characterization data of each compound.
Note: the coincidentally obtained single-crystals suitable for X-ray diffraction analysis of [(TIMMNMes*)FeV(NH*)(F)](PF6)3 (2e) were obtained only once (analogusly to the reaction procedure of 3) by changing the crystallization conditions to an SO2/HF mixture, thus, potentially hindering the HF elimination mechanism.
Crystal structure determination
Suitable single-crystals of the investigated compounds were embedded in protective perfluoropolyalkyether oil on a microscope slide, and a single specimen was selected and subsequently transferred to the cold nitrogen gas stream of the diffractometer. Intensity data were collected using MoKα radiation (λ = 0.71073 Å) on a Bruker Kappa PHOTON 2 IμS Duo diffractometer equipped with QUAZAR focusing Montel optics. Data were corrected for Lorentz and polarization effects, semi-empirical absorption corrections were performed on the basis of multiple scans using SADABS48. The structures were solved by direct methods (SHELX XT 2014/5)49 and refined by full-matrix least-squares procedures on F2 using SHELXL 2018/3 (ref. 50). All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms were placed in positions of optimized geometry, their isotropic displacement parameters were tied to those of the corresponding carrier atoms by a factor of either 1.2 or 1.5. Olex2 was used to prepare material for publication51 (see Supplementary Information for crystallographic data, data collection and structure refinement details).
Computational details
All computations were performed with ORCA v.5.0.1‒4 (refs. 37,38,39,52). The NPA charges were calculated with NBO v.7.0 (ref. 53), whereas the Bader charges were calculated with AIMAll v.19.10.12 (ref. 54). Orbitals and spin densities were visualized with IboView v.20211019-RevA55 or ChemCraft56. See the Supplementary Information for further details on DFT and CASSCF calculations.
Data availability
All data generated and analysed during this study are included in this article and its Supplementary Information, and are also available from the authors on reasonable request. Atomic coordinates and structure factors for the reported crystal structures have been deposited in the Cambridge Crystallographic Database under accession codes CCDC-2258913 for [(TIMMNMes)FeVI(N)(F)](PF6)2·CH2Cl2 (1·CH2Cl2); CCDC-2297600 for [(TIMMNMes*)FeV(NH*)(F)](PF6)3 (2e); and CCDC-2258914 for [(TIMMNMes*)FeV(=N*)(NCMe)](PF6)2MoF6·5 C2H3N (3·5 C2H3N). Source Data are provided with this paper.
References
Hohenberger, J., Ray, K. & Meyer, K. The biology and chemistry of high-valent iron–oxo and iron–nitrido complexes. Nat. Commun. 3, 720 (2012).
Costas, M., Mehn, M. P., Jensen, M. P. & Que, L. Jr. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 104, 939–986 (2004).
Hoffman, B. M., Dean, D. R. & Seefeldt, L. C. Climbing nitrogenase: toward a mechanism of enzymatic nitrogen fixation. Acc. Chem. Res. 42, 609–619 (2009).
Crossland, J. L. & Tyler, D. R. Iron–dinitrogen coordination chemistry: dinitrogen activation and reactivity. Coord. Chem. Rev. 254, 1883–1894 (2010).
McDonald, A. R. & Que, L. Jr. High-valent nonheme iron–oxo complexes: synthesis, structure, and spectroscopy. Coord.Chem. Rev. 257, 414–428 (2013).
Eikey, R. Nitrido and imido transition metal complexes of groups 6–8. Coord. Chem. Rev. 243, 83–124 (2003).
Saouma, C. T. & Peters, J. C. M≡E and M=E complexes of iron and cobalt that emphasize three-fold symmetry (E = O, N, NR). Coord. Chem. Rev. 255, 920–937 (2011).
Lu, J. B. et al. Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4–. Phys. Chem. Chem. Phys. 18, 31125–31131 (2016).
Schmidbaur, H. The history and the current revival of the oxo chemistry of iron in its highest oxidation states: FeVI–FeVIII. Z. Anorg. Allg. Chem. 644, 536–559 (2018).
Hunter, B. M. et al. Trapping an iron(VI) water-splitting intermediate in nonaqueous media. Joule 2, 747–763 (2018).
Sarma, R., Angeles-Boza, A. M., Brinkley, D. W. & Roth, J. P. Studies of the di-iron(VI) intermediate in ferrate-dependent oxygen evolution from water. J. Am. Chem. Soc. 134, 15371–15386 (2012).
Sharma, V. K., Chen, L. & Zboril, R. Review on high valent FeVI (ferrate): a sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain. Chem. Eng. 4, 18–34 (2015).
Vogel, C., Heinemann, F. W., Sutter, J., Anthon, C. & Meyer, K. An iron nitride complex. Angew. Chem. Int. Ed. 47, 2681–2684 (2008).
Scepaniak, J. J. et al. Structural and spectroscopic characterization of an electrophilic iron nitrido complex. J. Am. Chem. Soc. 130, 10515–10517 (2008).
Maity, A. K., Murillo, J., Metta-Magana, A. J., Pinter, B. & Fortier, S. A terminal iron(IV) nitride supported by a super bulky guanidinate ligand and examination of its electronic structure and reactivity. J. Am. Chem. Soc. 139, 15691–15700 (2017).
Scepaniak, J. J. et al. Synthesis, structure, and reactivity of an iron(V) nitride. Science 331, 1049–1052 (2011).
Keilwerth, M. et al. Ligand tailoring toward an air-stable iron(V) nitrido complex. J. Am. Chem. Soc. 143, 1458–1465 (2021).
Sabenya, G. et al. Generation, spectroscopic, and chemical characterization of an octahedral iron(V)–nitrido species with a neutral ligand platform. J. Am. Chem. Soc. 139, 9168–9177 (2017).
Betley, T. A. & Peters, J. C. A tetrahedrally coordinated L3Fe-Nx platform that accommodates terminal nitride (FeIV≡N) and dinitrogen (FeI–N2–FeI) ligands. J. Am. Chem. Soc. 126, 6252–6254 (2004).
Keilwerth, M. et al. From divalent to pentavalent iron imido complexes and an Fe(V) nitride via N–C bond cleavage. J. Am. Chem. Soc. 145, 873–887 (2023).
Thomas, C. M., Mankad, N. P. & Peters, J. C. Characterization of the terminal iron(IV) Imides {[PhBPtBu2(pz’)]FeIV≡NAd}+. J. Am. Chem. Soc. 128, 4956–4957 (2006).
Wang, L., Hu, L., Zhang, H., Chen, H. & Deng, L. Three-coordinate iron(IV) bisimido complexes with aminocarbene ligation: synthesis, structure, and reactivity. J. Am. Chem. Soc. 137, 14196–14207 (2015).
Hong, S. et al. A mononuclear nonheme iron(V)–imido complex. J. Am. Chem. Soc. 139, 8800–8803 (2017).
Tiago de Oliveira, F. et al. Chemical and spectroscopic evidence for an FeV–oxo complex. Science 315, 835–838 (2007).
Borrell, M., Andris, E., Navratil, R., Roithova, J. & Costas, M. Characterized cis-Fe(V)(O)(OH) intermediate mimics enzymatic oxidations in the gas phase. Nat. Commun. 10, 901 (2019).
Ghosh, M. et al. Formation of a room temperature stable Fe(V)(O) complex: reactivity toward unactivated C–H bonds. J. Am. Chem. Soc. 136, 9524–9527 (2014).
Fan, R. et al. Spectroscopic and DFT characterization of a highly reactive nonheme Fe(V)–oxo intermediate. J. Am. Chem. Soc. 140, 3916–3928 (2018).
Mondal, B., Neese, F., Bill, E. & Ye, S. Electronic structure contributions of non-heme oxo–iron(V) complexes to the reactivity. J. Am. Chem. Soc. 140, 9531–9544 (2018).
Rohde, J. U. et al. Crystallographic and spectroscopic characterization of a nonheme Fe(IV)=O complex. Science 299, 1037–1039 (2003).
Keilwerth, M. et al. A series of iron nitrosyl complexes {Fe-NO}6–9 and a fleeting {Fe-NO}10 intermediate en eoute to a metalacyclic iron nitrosoalkane. J. Am. Chem. Soc. 141, 17217–17235 (2019).
Citek, C., Oyala, P. H. & Peters, J. C. Mononuclear Fe(I) and Fe(II) acetylene adducts and their reductive protonation to terminal Fe(IV) and Fe(V) carbynes. J. Am. Chem. Soc. 141, 15211–15221 (2019).
Smith, J. M. Strongly donating scorpionate ligands. Comments Inorg. Chem. 29, 189–233 (2008).
Berry, J. F. et al. An octahedral coordination complex of iron(VI). Science 312, 1937–1941 (2006).
Martinez, J. L. et al. Structural and spectroscopic characterization of an Fe(VI) bis(imido) complex. Science 370, 356–359 (2020).
Neese, F. Prediction and interpretation of the 57Fe isomer shift in Mössbauer spectra by density functional theory. Inorg. Chim. Acta 337, 181–192 (2002).
DeBeer George, S., Petrenko, T. & Neese, F. Prediction of iron K-edge absorption spectra using time-dependent density functional theory. J. Phys. Chem. A 112, 12936–12943 (2008).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Neese, F. Software update: the ORCA program system—version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 12, e1606 (2022).
Neese, F. The SHARK integral generation and digestion system. J. Comput. Chem. 44, 381–396 (2023).
Long, A. K. M., Yu, R. P., Timmer, G. H. & Berry, J. F. Aryl C–H bond amination by an electrophilic diruthenium nitride. J. Am. Chem. Soc. 132, 12228–12230 (2010).
Schiller, C. et al. Cleavage of an aromatic C–C bond in ferrocene by insertion of an iridium nitrido nitrogen atom. J. Am. Chem. Soc. 145, 11392–11401 (2023).
Herber, R. H. & Johnson, D. Lattice dynamics and hyperfine interactions in M2FeO4 (M = K+, Rb+, Cs+) and M′FeO4 (M = Sr2+, Ba2+). Inorg. Chem. 18, 2786–1790, (1979).
Nieto, I., Ding, F., Bontchev, R. P., Wang, H. & Smith, J. M. Thermodynamics of hydrogen atom transfer to a high-valent iron imido complex. J. Am. Chem. Soc. 130, 2716–2717 (2008).
Fulmer, G. R. et al. NMR chemical shifts of trace impurities: common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 29, 2176–2179 (2010).
Ahrens, S., Peritz, A. & Strassner, T. Tunable aryl alkyl ionic liquids (TAAILs): the next generation of ionic liquids. Angew. Chem. Int. Ed. 48, 7908–7910 (2009).
Liu, H. Y., Scharbert, B. & Holm, R. H. An initial approach to biologically related bridged assemblies: pyridinethiolate-linked iron Fe4S4–Fe complex systems. J. Am. Chem. Soc. 113, 9529–9539 (1991).
Fluck, E. & Meiser, P. Tris(chlormethyl) amin und bis(chlormethyl)methylamin. Darstellung und chemische reaktionen. Chem. Ber. 106, 69–77 (1973).
Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 48, 3–10 (2015).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Neese, F. The ORCA program system. Comput. Mol. Sci. 2, 73–78 (2012).
Reed, A. E., Weinstock, R. B. & Weinhold, F. Natural population analysis. J. Chem. Phys. 83, 735–746 (1985).
Nakajima, T. & Hirao, K. The Douglas–Kroll–Hess approach. Chem. Rev. 112, 385–402 (2012).
Reiher, M. Relativistic Douglas–Kroll–Hess theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 139–149 (2011).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Acknowledgements
The work was supported by funds from the German Federal Ministry of Education and Research (BMBF support codes 03SF0502 and 03HY105I, K.M.) and the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). M.M. thanks the Freie Universität Berlin for their support and the German Research Foundation (DFG Project-ID 387284271–SFB 1349) for funding. W.M. acknowledges a research fellowship from the Alexander-von-Humboldt foundation. S.A.V.J. and S.D. thank the Max Planck Society for their support. The Fe K-edge HERFD XAS experiments were performed on beamline ID26 at the ESRF, Grenoble, France. We are grateful to V. Saveleva at the ESRF for providing assistance in using beamline ID26. Use of the Stanford Synchrotron Radiation Lightsource (SLAC) National Accelerator Laboratory is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. We gratefully acknowledge the scientific support and HPC resources provided by the Erlangen National High Performance Computing Center (NHR@FAU) of the FAU under NHR project n100af10. NHR funding is provided by federal and Bavarian state authorities. NHR@FAU hardware is partially funded by the German Research Foundation (DFG) (grant no. 440719683). This article is dedicated to Daniel J. Mindiola on the occasion of his 50th birthday.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
M.K. designed and performed the experiments, interpreted the results, crystallized compound 3 and drafted the manuscript. W.M. crystallized 1. M.M. performed the metal hexafluoride-related experiments, and crystallized intermediate 2e. S.A.V.J. and S.D. measured and interpreted the XAS data. K.B. and D.M. designed and performed the computational investigations, and interpreted the results. F.W.H. collected and refined the SC-XRD data of 1 and 3, and refined intermediate 2e. A.S. collected and interpreted the multinuclear NMR data. E.B. collected and interpreted the applied-field Mössbauer data. K.M. supervised the research project, discussed and interpreted the results, and edited and finalized the manuscript with all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Wei-Tsung Lee, Veronica Carta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Full Supplementary Information file (without xyz data, as requested).
Supplementary Data 1
Crystallographic data for compound 1; CCDC reference no. 2258913.
Supplementary Data 2
Crystallographic data for complex (intermediate) 2e; CCDC reference no. 2297600
Supplementary Data 3
Crystallographic data for complex 3; CCDC reference no. 2258914
Supplementary Data 4
The xyz coordinates for the optimized structures
Source data
Source Data Fig. 1
Zero- and applied-field Moessbauer and XAS data shown in Fig. 1.
Source Data Fig. 2
Zero-field Moessbauer and X-band EPR data shown in Fig. 2.
Source Data Fig. 3
Plots of isomer shift versus oxidation state and partial charge and XAS data shown in Fig. 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keilwerth, M., Mao, W., Malischewski, M. et al. The synthesis and characterization of an iron(VII) nitrido complex. Nat. Chem. 16, 514–520 (2024). https://doi.org/10.1038/s41557-023-01418-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01418-4
This article is cited by
-
Lifting iron higher and higher
Nature Chemistry (2024)