Abstract
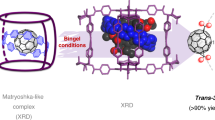
Self-assembled hosts, inspired by biological receptors and catalysts, show application potential in sustainable synthesis, energy conversion and medicine. Implementing multiple functionalities in the form of distinguishable building blocks, however, is difficult without risking narcissistic self-sorting or a statistical mess. Here we report a systematic series of integratively self-assembled heteroleptic cages in which two square-planar PdII cations are bridged by four different bis-pyridyl ligands, A, B, C and D, via synergistic effects to exclusively form a single isomer—the lantern-shaped cage [Pd2ABCD]. This self-sorting goal—forming just one out of 55 possible structures—is reached under full thermodynamic control and can be realized progressively (by combining progenitors, such as [Pd2A2C2] with [Pd2B2D2]), directly from ligands and PdII cations or by mixing all four corresponding homoleptic cages. The rational design of complex multicomponent assemblies that enables the modular incorporation of diverse chemical moieties will advance their applicability in functional nanosystems.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under the deposition numbers 2207621, trans-[Pd2A2B2](BF4)4; 2285849, [Pd2B2CD1](BF4)4; 2207623, [Pd2A2CD](BF4)4; 2285847, [Pd2B2D4C](BF4)4; 2285846, [Pd2ABCD](BF4)4; 2207626, [Pd2AB0CD](BF4)4; 2207627, [Pd2ABD4C](BF4)4; 2207628, [Pd2ABD2D](BF4)4; and 2285848, [Pd2ABCD2](BF4)4. Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the Article and its Supplementary Information, or from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Kelly, J. A., Sielecki, A. R., Sykes, B. D., James, M. N. G. & Phillips, D. C. X-ray crystallography of the binding of the bacterial cell wall trisaccharide NAM-NAG-NAM to lysozyme. Nature 282, 875–878 (1979).
Ringe, D. & Petsko, G. A. How enzymes work. Science 320, 1428–1429 (2008).
Liu, M. et al. Barely porous organic cages for hydrogen isotope separation. Science 366, 613–620 (2019).
Cook, T. R. & Stang, P. J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 115, 7001–7045 (2015).
Fujita, M. et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469–471 (1995).
Mal, P., Breiner, B., Rissanen, K. & Nitschke, J. R. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science 324, 1697–1699 (2009).
Zhang, D., Ronson, T. K., Zou, Y.-Q. & Nitschke, J. R. Metal–organic cages for molecular separations. Nat. Rev. Chem. 5, 168–182 (2021).
Galan, A. & Ballester, P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 45, 1720–1737 (2016).
Brown, C. J., Toste, F. D., Bergman, R. G. & Raymond, K. N. Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 115, 3012–3035 (2015).
Chen, L.-J., Yang, H.-B. & Shionoya, M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 46, 2555–2576 (2017).
Brzechwa-Chodzyńska, A., Drożdż, W., Harrowfield, J. & Stefankiewicz, A. R. Fluorescent sensors: a bright future for cages. Coord. Chem. Rev. 434, 213820 (2021).
Goeb, S. & Sallé, M. Electron-rich coordination receptors based on tetrathiafulvalene derivatives: controlling the host–guest binding. Acc. Chem. Res. 54, 1043–1055 (2021).
Wu, K. et al. The redox coupling effect in a photocatalytic RuII-PdII cage with TTF guest as electron relay mediator for visible-light hydrogen-evolving promotion. Angew. Chem. Int. Ed. 59, 2639–2643 (2020).
Wezenberg, S. J. Light-switchable metal-organic cages. Chem. Lett. 49, 609–615 (2020).
Li, R.-J., Tessarolo, J., Lee, H. & Clever, G. H. Multi-stimuli control over assembly and guest binding in metallo-supramolecular hosts based on dithienylethene photoswitches. J. Am. Chem. Soc. 143, 3865–3873 (2021).
Chakrabarty, R., Mukherjee, P. S. & Stang, P. J. Supramolecular coordination: self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 111, 6810–6918 (2011).
Fujita, D. et al. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 540, 563–566 (2016).
Wu, K., Tessarolo, J., Baksi, A. & Clever, G. H. Guest-modulated circularly polarized luminescence by ligand-to-ligand chirality transfer in heteroleptic Pdii coordination cages. Angew. Chem. Int. Ed. 61, e202205725 (2022).
Frank, M. et al. Light-induced charge separation in densely packed donor–acceptor coordination cages. J. Am. Chem. Soc. 138, 8279–8287 (2016).
García-Simón, C. et al. Enantioselective hydroformylation by a Rh-catalyst entrapped in a supramolecular metallocage. J. Am. Chem. Soc. 137, 2680–2687 (2015).
Wang, Q.-Q. et al. Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions. Nat. Chem. 8, 225–230 (2016).
Howlader, P., Das, P., Zangrando, E. & Mukherjee, P. S. Urea-functionalized self-assembled molecular prism for heterogeneous catalysis in water. J. Am. Chem. Soc. 138, 1668–1676 (2016).
Pruchyathamkorn, J. et al. A complex comprising a cyanine dye rotaxane and a porphyrin nanoring as a model light-harvesting system. Angew. Chem. Int. Ed. 59, 16455–16458 (2020).
Wang, W. et al. The construction of complex multicomponent supramolecular systems via the combination of orthogonal self-assembly and the self-sorting approach. Chem. Sci. 5, 4554–4560 (2014).
Kramer, R., Lehn, J. M. & Marquis-Rigault, A. Self-recognition in helicate self-assembly: spontaneous formation of helical metal complexes from mixtures of ligands and metal ions. Proc. Natl Acad. Sci. USA 90, 5394–5398 (1993).
Sauvage, J. P. & Weiss, J. Synthesis of biscopper(I) [3]-catenates: multiring interlocked coordinating systems. J. Am. Chem. Soc. 107, 6108–6110 (1985).
Kumazawa, K., Biradha, K., Kusukawa, T., Okano, T. & Fujita, M. Multicomponent assembly of a pyrazine-pillared coordination cage that selectively binds planar guests by intercalation. Angew. Chem. Int. Ed. 42, 3909–3913 (2003).
Zheng, Y.-R. et al. A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation. J. Am. Chem. Soc. 132, 16873–16882 (2010).
Wessjohann, L. A., Kreye, O. & Rivera, D. G. One-pot assembly of amino acid bridged hybrid macromulticyclic cages through multiple multicomponent macrocyclizations. Angew. Chem. Int. Ed. 56, 3501–3505 (2017).
He, Z., Jiang, W. & Schalley, C. A. Integrative self-sorting: a versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 44, 779–789 (2015).
Pullen, S., Tessarolo, J. & Clever, G. H. Increasing structural and functional complexity in self-assembled coordination cages. Chem. Sci. 12, 7269–7293 (2021).
Sun, Q.-F., Sato, S. & Fujita, M. An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly. Angew. Chem. Int. Ed. 53, 13510–13513 (2014).
Bloch, W. M. et al. Geometric complementarity in assembly and guest recognition of a bent heteroleptic cis-[Pd2LA2LB2] coordination cage. J. Am. Chem. Soc. 138, 13750–13755 (2016).
Sudan, S. et al. Identification of a heteroleptic Pd6L6L′6 coordination cage by screening of a virtual combinatorial library. J. Am. Chem. Soc. 143, 1773–1778 (2021).
Li, J.-R. & Zhou, H.-C. Bridging-ligand-substitution strategy for the preparation of metal-organic polyhedra. Nat. Chem. 2, 893–898 (2010).
Prusty, S., Yazaki, K., Yoshizawa, M. & Chand, D. K. A truncated molecular star. Chem. Eur. J. 23, 12456–12461 (2017).
Yamashina, M., Yuki, T., Sei, Y., Akita, M. & Yoshizawa, M. Anisotropic expansion of an M2L4 coordination capsule: host capability and frame rearrangement. Chem. Eur. J. 21, 4200–4204 (2015).
Chen, B., Holstein, J. J., Horiuchi, S., Hiller, W. G. & Clever, G. H. Pd(II) coordination sphere engineering: pyridine cages, quinoline bowls, and heteroleptic pills binding one or two fullerenes. J. Am. Chem. Soc. 141, 8907–8913 (2019).
Preston, D., Barnsley, J. E., Gordon, K. C. & Crowley, J. D. Controlled formation of heteroleptic [Pd2(La)2(Lb)2]4+ cages. J. Am. Chem. Soc. 138, 10578–10585 (2016).
Ogata, D. & Yuasa, J. Dynamic open coordination cage from nonsymmetrical imidazole–pyridine ditopic ligands for turn-on/off anion binding. Angew. Chem. Int. Ed. 58, 18424–18428 (2019).
Lewis, J. E. M., Tarzia, A., White, A. J. P. & Jelfs, K. E. Conformational control of Pd2L4 assemblies with unsymmetrical ligands. Chem. Sci. 11, 677–683 (2020).
Tessarolo, J., Lee, H., Sakuda, E., Umakoshi, K. & Clever, G. H. Integrative assembly of heteroleptic tetrahedra controlled by backbone steric bulk. J. Am. Chem. Soc. 143, 6339–6344 (2021).
De, S., Mahata, K. & Schmittel, M. Metal-coordination-driven dynamic heteroleptic architectures. Chem. Soc. Rev. 39, 1555–1575 (2010).
Johnson, A. M. & Hooley, R. J. Steric effects control self-sorting in self-assembled clusters. Inorg. Chem. 50, 4671–4673 (2011).
Liu, Y. et al. Controlled construction of heteroleptic [Pd2(LA)2(LB)(LC)]4+ cages: a facile approach for site-selective endo-functionalization of supramolecular cavities. Angew. Chem. Int. Ed. 62, e202217215 (2023).
Abe, T., Sanada. N., Takeuchi, K., Okazawa, A. & Hiraoka, S. Assembly of six types of heteroleptic Pd2L4 cages under kinetic control, J. Am. Chem. Soc. 146, (2024), https://doi.org/10.1021/jacs.3c09359
Wu, K., Zhang, B., Drechsler, C., Holstein, J. J. & Clever, G. H. Backbone-bridging promotes diversity in heteroleptic cages. Angew. Chem. Int. Ed. 60, 6403–6407 (2021).
Ebbert, K. E. et al. Resolution of minor size differences in a family of heteroleptic coordination cages by trapped ion mobility ESI-MS. Dalton Trans. 48, 11070–11075 (2019).
Acknowledgements
We thank the European Research Council (ERC Consolidator Grant 683083, RAMSES) for financial support. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy EXC 2033 ‘RESOLV’, project number 390677874, and GRK2376 ‘Confinement‐Controlled Chemistry’, project number 331085229. We thank B. Chen, J. Tessarolo, A. Platzek, K. Ebbert and S. Hasegawa for providing ligands and L. Schneider for ESI mass spectra measurements. We thank J. J. Holstein for helpful suggestions on the crystallographic data analysis.
Author information
Authors and Affiliations
Contributions
K.W. and G.H.C. conceived the project and wrote the manuscript together with E.B.; K.W. performed the experiments and analysed the data. A.B. provided ion mobility mass measurements. K.W. collected the X-ray single-crystal data and refined the crystal structures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Xiaopeng Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–308 and Tables 1–9.
Supplementary Data 1
Crystallographic data for trans-[Pd2A2B2](BF4)4 (CCDC reference 2207621).
Supplementary Data 2
Crystallographic data for [Pd2B2CD1](BF4)4 (CCDC reference 2285849).
Supplementary Data 3
Crystallographic data for [Pd2A2CD](BF4)4 (CCDC reference 2207623).
Supplementary Data 4
Crystallographic data for [Pd2B2D4C](BF4)4 (CCDC reference 2285847).
Supplementary Data 5
Crystallographic data for [Pd2ABCD](BF4)4 (CCDC reference 2285846).
Supplementary Data 6
Crystallographic data for [Pd2AB0CD](BF4)4 (CCDC reference 2207626).
Supplementary Data 7
Crystallographic data for [Pd2ABD4C](BF4)4 (CCDC reference 2207627).
Supplementary Data 8
Crystallographic data for [Pd2ABD2D](BF4)4 (CCDC reference 2207628).
Supplementary Data 9
Crystallographic data for [Pd2ABCD2](BF4)4 (CCDC reference 2285848).
Source data
Source Data Fig. 2
HR-ESI-MS of cage [Pd2ABCD]4+.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, K., Benchimol, E., Baksi, A. et al. Non-statistical assembly of multicomponent [Pd2ABCD] cages. Nat. Chem. 16, 584–591 (2024). https://doi.org/10.1038/s41557-023-01415-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01415-7