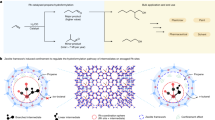
Abstract
In heterogeneous catalysis, the catalytic dehydrogenation reactions of hydrocarbons often exhibit a negative pressure dependence on hydrogen due to the competitive chemisorption of hydrocarbons and hydrogen. However, some catalysts show a positive pressure dependence for propane dehydrogenation, an important reaction for propylene production. Here we show that the positive activity dependence on H2 partial pressure of gallium oxide-based catalysts arises from metastable hydride mediation. Through in situ spectroscopic, kinetic and computational analyses, we demonstrate that under reaction conditions with H2 co-feeding, the dissociative adsorption of H2 on a partially reduced gallium oxide surface produces H atoms chemically bonded to coordinatively unsaturated Ga atoms. These metastable gallium hydride species promote C–H bond activation while inhibiting deep dehydrogenation. We found that the surface coverage of gallium hydride determines the catalytic performance. Accordingly, benefiting from proper H2 co-feeding, the alumina-supported, trace additive-modified gallium oxide catalyst GaOx–Ir–K/Al2O3 exhibited high activity and selectivity at high propane concentrations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data are available within the paper and its Supporting Information. The raw data for the Supplementary Figures are available in Supplementary Data 1. Source data are provided with this paper.
References
Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 295, 2053–2055 (2002).
Chen, M. et al. Small adsorbate-assisted shape control of Pd and Pt nanocrystals. Adv. Mater. 24, 862–879 (2012).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Zhang, X. et al. Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 3, 411–417 (2020).
Wu, C. H. et al. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2, 78–85 (2018).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2016).
Wang, H. et al. Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nat. Catal. 4, 418–424 (2021).
Li, J. et al. Self-adaptive dual-metal-site pairs in metal–organic frameworks for selective CO2 photoreduction to CH4. Nat. Catal. 4, 719–729 (2021).
Zhang, Z. et al. Ensembles of metastable states govern heterogeneous catalysis on dynamic interfaces. Acc. Chem. Res. 53, 447–458 (2020).
Liu, J. et al. Tackling CO poisoning with single-atom alloy catalysts. J. Am. Chem. Soc. 138, 6396–6399 (2016).
Jain, D. et al. Experimental and DFT investigation into chloride poisoning effects on nitrogen-coordinated iron–carbon (FeNC) catalysts for oxygen reduction reaction. J. Phys. Chem. C 124, 10324–10335 (2020).
Ramasamy, K. K. et al. Conversion of biomass-derived small oxygenates over HZSM-5 and its deactivation mechanism. Green Chem. 16, 748–760 (2014).
Ryoo, R. et al. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Zhou, H. et al. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 372, 76–80 (2021).
Zhang, T. et al. Synergistic mechanism of platinum–GaOx catalysts for propane dehydrogenation. Angew. Chem. Int. Ed. 61, e202201453 (2022).
Sattler, J. J. et al. Platinum-promoted Ga/Al2O3 as highly active, selective, and stable catalyst for the dehydrogenation of propane. Angew. Chem. Int. Ed. 53, 9251–9256 (2014).
Castro-Fernández, P. et al. Propane dehydrogenation on Ga2O3-based catalysts: contrasting performance with coordination environment and acidity of surface sites. ACS Catal. 11, 907–924 (2021).
Sattler, J. J. H. B. et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Liu, Y. et al. Periodic density functional theory study of propane dehydrogenation over perfect Ga2O3(100) surface. J. Phys. Chem. C 112, 20382–20392 (2008).
Chang, Q.-Y. et al. Rational design of single-atom-doped Ga2O3 catalysts for propane dehydrogenation: breaking through volcano plot by Lewis acid–base interactions. ACS Catal. 11, 5135–5147 (2021).
Jochum, W. et al. Hydrogen on polycrystalline β-Ga2O3: surface chemisorption, defect formation, and reactivity. J. Catal. 256, 268–277 (2008).
Pan, Y.-X. et al. Hydrogen adsorption on Ga2O3 surface: a combined experimental and computational study. J. Phys. Chem. C 115, 10140–10146 (2011).
Galvita, V. et al. Ethane dehydrogenation on Pt/Mg(Al)O and PtSn/Mg(Al)O catalysts. J. Catal. 271, 209–219 (2010).
Siddiqi, G. et al. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 274, 200–206 (2010).
Wu, J. et al. n-Butane dehydrogenation over Pt/Mg(In)(Al)O. Appl. Catal. A 470, 208–214 (2014).
Saerens, S. et al. The positive role of hydrogen on the dehydrogenation of propane on Pt(111). ACS Catal. 7, 7495–7508 (2017).
Yang, M.-L. et al. Tuning selectivity and stability in propane dehydrogenation by shaping Pt particles: a combined experimental and DFT study. J. Mol. Catal. A 395, 329–336 (2014).
Li, Q. et al. Kinetics of propane dehydrogenation over Pt–Sn/Al2O3 catalyst. Appl. Catal. A 398, 18–26 (2011).
Nykänen, L. et al. Selectivity in propene dehydrogenation on Pt and Pt3Sn surfaces from first principles. ACS Catal. 3, 3026–3030 (2013).
Dai, Y. et al. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 50, 5590–5630 (2021).
Zhang, Y. et al. Control of coordinatively unsaturated Zr sites in ZrO2 for efficient C–H bond activation. Nat. Commun. 9, 3794 (2018).
Otroshchenko, T. et al. Effect of hydrogen and supported metal on selectivity and on-stream stability of ZrO2-based catalysts in non-oxidative propane dehydrogenation. Catal. Commun. 144, 106068 (2020).
Nishi, K. et al. Deconvolution analysis of Ga K-edge XANES for quantification of gallium coordinations in oxide environments. J. Phys. Chem. B 102, 10190–10195 (1998).
Phadke, N. M. et al. Characterization of isolated Ga3+ cations in Ga/H-MFI prepared by vapor-phase exchange of H-MFI zeolite with GaCl3. ACS Catal. 8, 6106–6126 (2018).
Getsoian, A. et al. Organometallic model complexes elucidate the active gallium species in alkane dehydrogenation catalysts based on ligand effects in Ga K-edge XANES. Catal. Sci. Technol. 6, 6339–6353 (2016).
Meitzner, G. D. et al. The chemical state of gallium in working alkane dehydrocyclodimerization catalysts. In situ gallium K-edge X-ray absorption spectroscopy. J. Catal. 140, 209–225 (1993).
Serykh, A. I. et al. In situ X-ray photoelectron spectroscopy study of gallium-modified MFI zeolite. Surf. Sci. 603, 2037–2041 (2009).
Cybulskis, V. J. et al. The nature of the isolated gallium active center for propane dehydrogenation on Ga/SiO2. Catal. Lett. 147, 1252–1262 (2017).
Collins, S. et al. Gallium–hydrogen bond formation on gallium and gallium–palladium silica-supported catalysts. J. Catal. 211, 252–264 (2002).
Collins, S. E. et al. Hydrogen chemisorption on gallium oxide polymorphs. Langmuir 21, 962–970 (2005).
Zhao, Y. et al. Raman spectroscopy and characterisation of α-gallium oxyhydroxide and β-gallium oxide nanorods. J. Raman Spectrosc. 39, 1494–1501 (2008).
Reuter, K. et al. First-principles atomistic thermodynamics for oxidation catalysis: surface phase diagrams and catalytically interesting regions. Phys. Rev. Lett. 90, 046103 (2003).
Otroshchenko, T. et al. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 50, 473–527 (2021).
Schreiber, M. W. et al. Lewis–Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes. J. Am. Chem. Soc. 140, 4849–4859 (2018).
Yuan, Y. et al. Ga speciation in Ga/H-ZSM-5 by in-situ transmission FTIR spectroscopy. J. Catal. 393, 60–69 (2021).
Mansoor, E. et al. Computational modeling of the nature and role of Ga species for light alkane dehydrogenation catalyzed by Ga/H-MFI. ACS Catal. 8, 6146–6162 (2018).
Phadke, N. M. et al. Mechanism and kinetics of propane dehydrogenation and cracking over Ga/H-MFI prepared via vapor-phase exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 141, 1614–1627 (2019).
Zhao, Z. J. et al. Hydroxyl-mediated non-oxidative propane dehydrogenation over VOx/γ-Al2O3 catalysts with improved stability. Angew. Chem. Int. Ed. 57, 6791–6795 (2018).
Fu, H. et al. Periodic density functional theory study of propane oxidative dehydrogenation over V2O5(001) surface. J. Am. Chem. Soc. 128, 11114–11123 (2006).
Deringer, V. L. et al. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Dunnington, B. D. & Schmidt, J. R. Generalization of natural bond orbital analysis to periodic systems: applications to solids and surfaces via plane-wave density functional theory. J. Chem. Theory Comput. 8, 1902–1911 (2012).
Fridman, V. Catalyst for dehydrogenation of hydrocarbons. Sud-Chemie Inc. US patent 8101541B2 (2012).
Acknowledgements
We acknowledge the National Key R&D Program of China (2021YFA1501302), the National Natural Science Foundation of China (22121004, U1862207, and 22122808), the Haihe Laboratory of Sustainable Chemical Transformations (CYZC202107, the Program of Introducing Talents of Discipline to Universities (BP0618007) and the XPLORER PRIZE for financial support. We also thank the staff at the 1W1B beamline of the Beijing Synchrotron Radiation Facility for help in characterization and computing resources at High Performance Computing Center of Tianjin University.
Author information
Authors and Affiliations
Contributions
J.G. conceived and supervised the research. G.S. contributed to catalyst synthesis and characterization. G.S., T.Z. and K.T. carried out catalytic performance tests. G.S., S.C. and L.Z. performed the XAS measurements and analysed the data. L.L. X.C., S.S. and Z.-J.Z. carried out DFT calculations. G.S., Z.-J.Z., C.P., S.C. and J.G. wrote the paper. All authors participated in the discussion of the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Tzonka Mineva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Catalytic performance of CrOx-K/Al2O3 and GaOx/Al2O3.
a,b, Catalytic performance of CrOx-K/Al2O3 with different inlet H2 concentrations at an inlet C3H8 concentration of 40 vol% (a) and the corresponding initial (after 5 min reaction) catalytic performance (b). Catalytic testing conditions: 600 °C, atmospheric pressure, 200 mg catalyst, WHSV of propane = 11.8 h−1, 40 vol% C3H8, 0–40 vol% H2, 50 ml min−1 total flow rate balanced with N2. c, Comparison of the initial rate of propylene formation normalized by catalyst mass between GaOx/Al2O3 at an inlet H2/C3H8 ratio of 1.0 and a commercial CrOx-K/Al2O3 analogue without co-feeding H2. Catalytic testing conditions: 600 °C, atmospheric pressure, 200 mg catalyst, WHSV of propane = 11.8 h−1, 40 vol% C3H8, 0 or 40 vol% H2, 50 ml min−1 total flow rate balanced with N2. d,e, Catalytic performance of GaOx/Al2O3 at different inlet C3H8 concentrations with constant WHSV and inlet H2/C3H8 ratios (d) and the corresponding initial catalytic performance (e). Catalytic testing conditions: 600 °C, atmospheric pressure, 50–200 mg catalyst, WHSV of propane = 11.8 h−1, 10–40 vol% C3H8, H2/C3H8 = 1.0, 50 ml min−1 total flow rate balanced with N2. f-i, Catalytic performance of Ga2O3/Al2O3 and GaOx/Al2O3 with different inlet H2 concentrations (f) (h) and the corresponding initial catalytic performance (g) (i). Catalytic testing conditions: 600 °C, atmospheric pressure, 50 mg (f) (h) or 200 mg (g) (i) catalyst, WHSV of propane = 47.2 h−1 (f) (h) or 11.8 h−1 (g) (i), 40 vol% C3H8, 0–40 vol% H2, 50 ml min−1 total flow rate balanced with N2.
Extended Data Fig. 2 C-H bond activation and apparent activation energy.
a, Mass spectral (MS) signals of C3H8, C3H6 and H2 (m/z equals 41, 29 and 2, respectively) in TPSR experiments for the fresh (Ga2O3/Al2O3) and pre-reduced (GaOx/Al2O3) catalysts. During the pre-reduction, the fresh catalyst was first treated with 10% H2/Ar at 600 °C for 1 h and then purged with Ar. b, Experimental Arrhenius plots of GaOx/Al2O3 with different inlet H2/C3H8 ratios, based on the increase in rate of propylene formation due to H2 co-feeding at the corresponding temperature. Δr = r(with H2 co-feeding) - r(without H2 co-feeding). Catalytic testing conditions: 570–600 °C, atmospheric pressure, 25 mg catalyst, WHSV of propane = 33.4 h−1, 7 ml min−1 C3H8, H2/C3H8 = 0.5, 1.0, 1.5, and 2.0, 50 ml·min−1 total flow rate balanced with N2. c,d, CH4-TPSR results of GaOx/Al2O3 with or without co-feeding D2. For CH4, H2, D2, HD, CH3D, CH2D2, CHD3 and CD4, m/e equals 16, 2, 4, 3, 17, 18, 19 and 20, respectively.
Extended Data Fig. 3 Evolution of catalyst surface structure.
a, In situ DRIFTS spectra of the alumina support treated with D2 at 600 °C. The sample was first heated at 600 °C in Ar and then the background spectrum was collected. After that, D2 was introduced into the DRIFTS cell. Upon treatment with D2, the negative bands at 3768–3678 cm−1 and the positive bands at 2770–2707 cm−1 appeared simultaneously, displaying the expected H-D isotopic exchange for the AlO-H bands. b,c, In situ DRIFTS spectra of GaOx/Al2O3 exposed to H2 (b) or D2 (c) at 600 °C. d, In situ DRIFTS spectra of GaOx/Al2O3 at 600 °C with different H2 partial pressures. e, The dependence of the intensity of gallium hydride band on H2 partial pressure. f, In situ Raman spectra of Ga2O3/Al2O3 during successive treatments with different gases at 600 °C. g, Schematic representation of the evolution of surface structure of gallium oxide supported by alumina during treatment with different gases.
Supplementary information
Supplementary Information
Supplementary Discussion and Supplementary Figs. 1–12, and Tables 1–11.
Supplementary Data 1
Raw data for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 1a
Unprocessed TEM image.
Source Data Extended Data Fig 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, G., Zhao, ZJ., Li, L. et al. Metastable gallium hydride mediates propane dehydrogenation on H2 co-feeding. Nat. Chem. 16, 575–583 (2024). https://doi.org/10.1038/s41557-023-01392-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01392-x
This article is cited by
-
Boosting propane dehydrogenation by gallium hydride species formed in situ
Science China Chemistry (2024)
-
Optimizing propylene selectivity and stability over Pt–Sn/MgAl2O4 catalysts for propane dehydrogenation
Journal of Porous Materials (2024)
-
Surprised by exceptional stability of confined single-atom cluster catalysts
Science China Materials (2024)