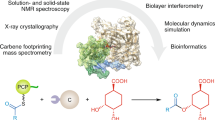
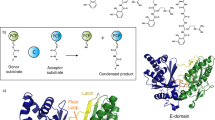
Abstract
Many peptide-derived natural products are produced by non-ribosomal peptide synthetases (NRPSs) in an assembly-line fashion. Each amino acid is coupled to a designated peptidyl carrier protein (PCP) through two distinct reactions catalysed sequentially by the single active site of the adenylation domain (A-domain). Accumulating evidence suggests that large-amplitude structural changes occur in different NRPS states; yet how these molecular machines orchestrate such biochemical sequences has remained elusive. Here, using single-molecule Förster resonance energy transfer, we show that the A-domain of gramicidin S synthetase I adopts structurally extended and functionally obligatory conformations for alternating between adenylation and thioester-formation structures during enzymatic cycles. Complementary biochemical, computational and small-angle X-ray scattering studies reveal interconversion among these three conformations as intrinsic and hierarchical where intra-A-domain organizations propagate to remodel inter-A–PCP didomain configurations during catalysis. The tight kinetic coupling between structural transitions and enzymatic transformations is quantified, and how the gramicidin S synthetase I A-domain utilizes its inherent conformational dynamics to drive directional biosynthesis with a flexibly linked PCP domain is revealed.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All smFRET trajectories (photon arrival times), SAXS raw intensity and P(r) files and MD simulations (PSF and CHARMM DCD files) are available at https://doi.org/10.34770/6ehx-sr32. All other biochemical data are reported in the Supplementary Tables and Figures or Extended Data Figures with source data files provided in this paper. The following protein structures were used for computational modelling and MD simulations: PDB:1AMU, PDB:1ULT, PDB:3C5E, PDB:4FUQ, PDB:5ES5, PDB:5ES8, PDB:5ES9 and PDB:6MFX. Source data are provided with this paper.
Code availability
Source codes for the photon-by-photon MIM analysis17, motional narrowing analysis40 and bash codes interfaced with a portable batch system are publicly available at https://github.com/PrincetonUniversity/smFRET_MaximumInformationMethod. These codes are generalizable and were not custom made for this study.
References
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459 (2015).
Wang, Z., Koirala, B., Hernandez, Y., Zimmerman, M. & Brady, S. F. Bioinformatic prospecting and synthesis of a bifunctional lipopeptide antibiotic that evades resistance. Science 376, 991–996 (2022).
Marahiel, M. A., Stachelhaus, T. & Mootz, H. D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97, 2651–2674 (1997).
Fischbach, M. A. & Walsh, C. T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106, 3468–3496 (2006).
Sussmuth, R. D. & Mainz, A. Nonribosomal peptide synthesis—principles and prospects. Angew. Chem. Int. Ed. 56, 3770–3821 (2017).
Conti, E., Stachelhaus, T., Marahiel, M. A. & Brick, P. Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S. EMBO J. 16, 4174–4183 (1997).
Gulick, A. M., Starai, V. J., Horswill, A. R., Homick, K. M. & Escalante-Semerena, J. C. The 1.75 Å crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A. Biochemistry 42, 2866–2873 (2003).
Yonus, H. et al. Crystal structure of DltA. Implications for the reaction mechanism of non-ribosomal peptide synthetase adenylation domains. J. Biol. Chem. 283, 32484–32491 (2008).
Reger, A. S., Carney, J. M. & Gulick, A. M. Biochemical and crystallographic analysis of substrate binding and conformational changes in acetyl-CoA synthetase. Biochemistry 46, 6536–6546 (2007).
Gulick, A. M. Conformational dynamics in the acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem. Biol. 4, 811–827 (2009).
Reimer, J. M., Aloise, M. N., Harrison, P. M. & Schmeing, T. M. Synthetic cycle of the initiation module of a formylating nonribosomal peptide synthetase. Nature 529, 239–242 (2016).
Reimer, J. M. et al. Structures of a dimodular nonribosomal peptide synthetase reveal conformational flexibility. Science 366, eaaw4388 (2019).
Drake, E. J. et al. Structures of two distinct conformations of holo-non-ribosomal peptide synthetases. Nature 529, 235–238 (2016).
Wang, J. et al. Catalytic trajectory of a dimeric nonribosomal peptide synthetase subunit with an inserted epimerase domain. Nat. Commun. 13, 592 (2022).
Alfermann, J. et al. FRET monitoring of a nonribosomal peptide synthetase. Nat. Chem. Biol. 13, 1009–1015 (2017).
Mayerthaler, F. et al. Intermediary conformations linked to the directionality of the aminoacylation pathway of nonribosomal peptide synthetases. RSC Chem. Biol. 2, 843–854 (2021).
Watkins, L. P. & Yang, H. Information bounds and optimal analysis of dynamic single molecule measurements. Biophys. J. 86, 4015–4029 (2004).
Watkins, L. P., Chang, H. & Yang, H. Quantitative single-molecule conformational distributions: a case study with poly-(l-proline). J. Phys. Chem. A 110, 5191–5203 (2006).
Boehr, D. D., Dyson, H. J. & Wright, P. E. An NMR perspective on enzyme dynamics. Chem. Rev. 106, 3055–3079 (2006).
Bellissent-Funel, M.-C. et al. Water determines the structure and dynamics of proteins. Chem. Rev. 116, 7673–7697 (2016).
Alderson, T. R. & Kay, L. E. NMR spectroscopy captures the essential role of dynamics in regulating biomolecular function. Cell 184, 577–595 (2021).
Gopich, I. V. & Szabo, A. Single-macromolecule fluorescence resonance energy transfer and free-energy profiles. J. Phys. Chem. B 107, 5058–5063 (2003).
Zettler, J. & Mootz, H. D. Biochemical evidence for conformational changes in the cross-talk between adenylation and peptidyl-carrier protein domains of nonribosomal peptide synthetases. FEBS J. 277, 1159–1171 (2010).
Kochan, G., Pilka, E. S., von Delft, F., Oppermann, U. & Yue, W. W. Structural snapshots for the conformation-dependent catalysis by human medium-chain acyl-coenzyme A synthetase ACSM2A. J. Mol. Biol. 388, 997–1008 (2009).
Sun, X., Li, H., Alfermann, J., Mootz, H. D. & Yang, H. Kinetics profiling of gramicidin S synthetase A, a member of nonribosomal peptide synthetases. Biochemistry 53, 7983–7989 (2014).
Bernado, P., Mylonas, E., Petoukhov, M. V., Blackledge, M. & Svergun, D. I. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 129, 5656–5664 (2007).
Tria, G., Mertens, H. D., Kachala, M. & Svergun, D. I. Advanced ensemble modelling of flexible macromolecules using X-ray solution scattering. IUCrJ 2, 207–217 (2015).
Meisburger, S. P. et al. Domain movements upon activation of phenylalanine hydroxylase characterized by crystallography and chromatography-coupled amall-angle X-ray scattering. J. Am. Chem. Soc. 138, 6506–6516 (2016).
Gulick, A. M. Structural insight into the necessary conformational changes of modular nonribosomal peptide synthetases. Curr. Opin. Chem. Biol. 35, 89–96 (2016).
Reimer, J. M., Haque, A. S., Tarry, M. J. & Schmeing, T. M. Piecing together nonribosomal peptide synthesis. Curr. Opin. Struct. Biol. 49, 104–113 (2018).
Miller, B. R., Sundlov, J. A., Drake, E. J., Makin, T. A. & Gulick, A. M. Analysis of the linker region joining the adenylation and carrier protein domains of the modular nonribosomal peptide synthetases. Proteins 82, 2691–2702 (2014).
Ruschenbaum, J., Steinchen, W., Mayerthaler, F., Feldberg, A. L. & Mootz, H. D. FRET monitoring of a nonribosomal peptide synthetase elongation module reveals carrier protein shuttling between catalytic domains. Angew. Chem. Int. Ed. 61, e202212994 (2022).
Tompa, P. The principle of conformational signaling. Chem. Soc. Rev. 45, 4252–4284 (2016).
Yogurtcu, O. N., Wolgemuth, C. W. & Sun, S. X. Mechanical response and conformational amplification in α-helical coiled coils. Biophys. J. 99, 3895–3904 (2010).
Ma, B., Tsai, C.-J., Haliloğlu, T. & Nussinov, R. Dynamic allostery: linkers are not merely flexible. Structure 19, 907–917 (2011).
Herbst, D. A., Townsend, C. A. & Maier, T. The architectures of iterative type I PKS and FAS. Nat. Prod. Rep. 35, 1046–1069 (2018).
Grininger, M. Enzymology of assembly line synthesis by modular polyketide synthases. Nat. Chem. Biol. 19, 401–415 (2023).
Luo, L., Burkart, M. D., Stachelhaus, T. & Walsh, C. T. Substrate recognition and selection by the initiation module PheATE of gramicidin S synthetase. J. Am. Chem. Soc. 123, 11208–11218 (2001).
Clegg, R. M. Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol 211, 353–388 (1992).
Hanson, J. A. et al. Illuminating the mechanistic roles of enzyme conformational dynamics. Proc. Natl Acad. Sci. USA 104, 18055–18060 (2007).
Hanson, J. A. & Yang, H. A general statistical test for correlations in a finite-length time series. J. Chem. Phys. 128, 214101 (2008).
Hanson, J. A. & Yang, H. Quantitative evaluation of cross correlation between two finite-length time series with applications to single-molecule FRET. J. Phys. Chem. B 112, 13962–13970 (2008).
Yang, H. The orientation factor in single-molecule Forster-type resonance energy transfer, with examples for conformational transitions in proteins. Isr. J. Chem. 49, 313–321 (2009).
Watkins, L. P. & Yang, H. Detection of intensity change points in time-resolved single-molecule measurements. J. Phys. Chem. B 109, 617–628 (2005).
Silverman, B. W. Weak and strong uniform consistency of kernel estimate of a density and its derivatives. Ann. Stat. 6, 177–184 (1978).
Silverman, B. W. Density Estimation for Statistics and Data Analysis (Chapman & Hall, 1986).
Press, W. H., Teukolsky, S. A., Vetterling, W. T. & Flannery, B. P. Numerical Recipes in C++: The Art of Scientific Computing, 2 edn (Cambridge Univ. Press, 2002).
Kubo, R. & Tomita, K. A general theory of magnetic resonance absorption. J. Phys. Soc. Jpn. 9, 888–919 (1954).
Anderson, P. W. A mathematical model for the narrowing of spectral lines by exchange or motion. J. Phys. Soc. Jpn. 9, 316–339 (1954).
Geva, E. & Skinner, J. L. Two-state dynamics of single biomolecules in solution. Chem. Phys. Lett. 288, 225–229 (1998).
DiCiccio, T. J. & Efron, B. Bootstrap confidence intervals. Stat. Sci. 11, 189–212 (1996).
Efron, B. 1977 Rietz lecture—bootstrap methods—another look at the jackknife. Ann. Stat. 7, 1–26 (1979).
Yang, H. in Theory and Evaluation of Single-Molecule Signals (eds Barkai, E., Brown, F. L. H., Orrit, M. & Yang, H.) 1–29 (World Scientific, 2008).
Hanson, J. A., Brokaw, J., Hayden, C. C., Chu, J. W. & Yang, H. Structural distributions from single-molecule measurements as a tool for molecular mechanics. Chem. Phys. 396, 61–71 (2012).
Roy, R., Hohng, S. & Ha, T. A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 (2008).
Cover, T. M. & Thomas, J. A. Elements of Information Theory. (John Wiley, 1991).
Skilling, J. & Bryan, R. K. Maximum entropy image reconstruction: general algorithm. Mon. Not R. Astron. Soc. 211, 111–124 (1984).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all‐atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Compt. Phys. 23, 327–341 (1977).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Nosé, S. & Klein, M. L. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 50, 1055–1076 (1983).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Feller, S. E., Zhang, Y., Pastor, R. W. & Brooks, B. R. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Skou, S., Gillilan, R. E. & Ando, N. Synchrotron-based small-angle X-ray scattering of proteins in solution. Nat. Protoc. 9, 1727–1739 (2014).
Svergun, D. I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 (1992).
Svergun, D., Barberato, C. & Koch, M. H. J. CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 28, 768–773 (1995).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Petrey, D. et al. Using multiple structure alignments, fast model building, and energetic analysis in fold recognition and homology modeling. Proteins 53, 430–435 (2003).
Acknowledgements
This work was supported by an HFSP award (number RGP0031/2010-C202, to H.D.M., T.K. and H.Y.), the Deutsche Forschungsgemeinschaft (DFG MO1073/8-1, to H.D.M.) and Princeton University (to H.Y.). The US NIH (GM124847) and Princeton University (start-up funds) are acknowledged for financial support (to N.A.). Also acknowledged are support (to J.-W.C.) from Taiwan MOST (109-2113-M-009-023-) and MOE (SPDSBTD project and IDS2B centre, Featured Areas Research Centre Program). The US NSF is acknowledged for a Graduate Research Fellowship (DGE-0646086, to T.E.M.). SAXS was conducted at the Centre for High-Energy X-ray Sciences (CHEXS), which is supported by the NSF (BIO, ENG and MPS Directorates) under award DMR-1829070, and the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award P30 GM124166 from the National Institute of General Medical Sciences. This work used the Extreme Science and Engineering Discovery Environment Resource Kraken at the NICS through allocation MCB140041, supported by NSF grant number ACI-1548562. Also used were resources of the National Energy Research Scientific Computing Centre, a US Department of Energy Office of Science User Facility operated under contract number DE-AC02-05CH11231. We thank A. Choi, E. Dehling and L. Y. Zhang for their efforts during the early phase of this project. We also thank T. W. Muir and D. Fiedler for the use of a stopped-flow fluorometer and HPLC, respectively, and J. Eng for technical support with mass spectrometry.
Author information
Authors and Affiliations
Contributions
H.Y. and H.D.M. conceived the study. X.S., H.Y., J.A. and H.D.M. planned the experiments. X.S. and H.L. performed smFRET and bulk-FRET experiments. X.S., J.A., H.L. and F.M. performed biochemical experiments. Y.-T.C, T.E.M. and J.-W.C. performed computational modelling and MD simulations. M.B.W., N.A. and H.L. performed SEC-SAXS experiments which were analysed by M.B.W. and N.A. X.S., H.Y., J.A., H.D.M., M.B.W., N.A., C.-Y.W. and T.K. interpreted the data. X.S., C.-Y.W., H.Y. and N.A. wrote the manuscript. All authors participated in discussion and manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Workflow to integrate X-ray structures, smFRET, bulk-FRET kinetics analysis, MD simulations and SAXS ensemble modelling.
Panels a–f summarize the smFRET workflow showing selection of smFRET constructs (a), determination of analysis methods (b), single-molecule data-pruning steps (c), maximum-information analysis (d), motional narrowing analysis (e) and smFRET constraints including peak positions of dye–dye distances and interconversion kinetic rates (f). For the trajectory shown in (d), the distance uncertainty was α = 0.11, which corresponded to an average time resolution of 〈∆〉 = 3.3 ms. For (e), at ∆=2.3 ms, the GrsA conformational PDF clearly displays two peaks. As the time resolution decreases (longer ∆), the two peaks coalesce into one at ∆ ≳5.1 ms. This motional-narrowing phenomenon shows that the timescales of GrsA A-domain conformational dynamics overlap with those of smFRET experiment data acquisition. For (f1–f2), a quartile outlier test indicated that individual differences between smFRET measurements and bulk-FRET (f1) and those between smFRET measurements and unconstrained MD models (f2) are statistical. Panels g–h describe the bulk-FRET and smFRET integration and the associated kinetics modelling that determine the chemical state assignment, kinetic scheme, populations and rates. Panels i–j show the integration of smFRET and MD simulations. Panel k shows the smFRET and SAXS integration. One 10,000-structure pool was generated per A-domain conformation and each point in the cloud plot represents the maximum distance from any atom in the PCP domain to the Cα of residue 527 at the C-terminus of the AC-subdomain. The distance distribution is for the distance from the aforementioned PCP position to the NE atom of W239 located inside the Phe-binding pocket. Different combinations of pools were used to test the ability of various A-domain conformations to allow for sufficient conformational sampling of the PCP domain to explain the experimental data.
Extended Data Fig. 2 Biochemical and biophysical control experiments show that the labelled GrsA mutants are functional, and that the dipoles of attached dyes can randomize on the millisecond timescale.
a, Similar thioester formation activities measured by ESI-TOF MS for holo cysteine-free A-PCP (hCF), labelled hCC1 and labelled hCC2. Data plotted are percentages of the first Phe-adduct mass peak and presented as mean ± 1 s.d. from three time-dependent FRET kinetic runs for hCF and labelled hCC1, and two runs for labelled hCC2. Solid lines are single exponential functions with fitting results labelled. b–c, Binding to Phe-AMS, a non-hydrolysable Phe-AMP mimic, results in a more compact state as shown by a native PAGE mobility assay for CC1 (b) and CC2 (c) in both the apo and holo forms. n = 2 in (b) and n = 4 in (c) for independently repeated native PAGE analyses with similar results. d, The His-tag and anti–His-tag antibody interaction does not affect the thioester formation activity of hCF probed by ESI-TOF MS. Mass spectra were normalized to the maximum peak height. The relative ESI-TOF MS peak height was used in calculating the relative activity between the sample of 1:1 hCF:anti-His-tag antibody and the hCF alone. The relative activity is shown as mean ± 1 s.d. from three kinetic runs with 90-s incubation. Inset: a cartoon representation of the immobilization scheme. e, Low bulk anisotropy during FRET process was observed for aCC2-K434A and hCC2-K434A with or without substrates. Data are presented as mean ± 1 s.d. from four repeats of hCC2-K434A with substrates and three repeats for other conditions. f–k, Representative autocorrelation functions of the acceptor channel (f, g), the donor channel (h, i) and the cross-correlation functions between the two channels during smFRET (j, k). Panels (f), (h) and (j) are for single aCC1molecules, and (g), (i) and (k) are for single aCC2-R439A molecules. The excitation was linearly polarized and modulated at 1 kHz so that any unwanted interaction between attached dyes and proteins on millisecond or slower would have obvious signatures in these correlation tests at the oscillation frequency of 1 kHz. However, no significant correlation at such lag time was observed; therefore, these results ruled out the possibility that dyes are transiently immobilized on the protein surface on millisecond or slower time scale. Dashed lines represent 95% confidence level.
Extended Data Fig. 3 smFRET studies of GrsA show that the high FRET states in both CC1 and CC2 are from the T-conformation involving the conserved K434 and R439.
a, Sequence conservation of the A8 motif including the conserved K434 and R439 generated by WebLogo (v2.8.2)71. b, Close-up of the active site containing Phe-AMP and the interacting A8 motif revealed by MD simulations. K434 and R439 likely interact with α-phosphate of Phe-AMP in T-state. Inset: location of the close-up from the T-state A-domain. c–l, Conformational distributions, and assignments of the three states (A-, T-, and X-states), in the absence (black) or the presence (salmon) of 100-µM Phe-AMS for aCC2 (c), aCC2-K434A (d), aCC2-R439A (e), aCC1 (f), aCC1-R439A (g), hCC2 (h), hCC2-K434A (i), hCC2-R439A (j), hCC1 (k) and hCC1-R439A (l). For comparison, smFRET PDF of parental controls with 100-µM Phe-AMS are plotted as grey lines in corresponding panels (d, e, g, i, j, l). In (c–l) top panels, the solid lines are conformational probability density functions extracted by maximum-entropy deconvolution and the dashed lines are distribution error bounds that are 1 s.d. from 25 bootstrapped smFRET distributions. Bottom: relative populations with vertical error bars, P(%), smFRET peak positions (uncertainty indicated by translucent vertical bars’ thickness) and normalized transition rates (horizontal arrows). Centres of peak positions and populations are shown as motional narrowing fits of nine time-resolved smFRET conformational distributions and errors are 1 s.d. of motional narrowing fits from 25 bootstrapped smFRET datasets. Phe-AMS favours the high-FRET population in CC1 or CC2 but not in mutants with K434A or R439A, compared to the corresponding substrate-free data set for each mutant.
Extended Data Fig. 4 MD-equilibrated A- and T- conformations are similar to the corresponding X-ray structures.
a–d, Structural superpositions between the X-ray (slate, PDB 1AMU in A-conformation and 5ES8-based homology model for T-conformation) and two unconstrained MD-equilibrated A-domain conformations (grey, A-conformation in a–b and T-conformation in c–d) with attached dyes in sticks. MD-modelled A- and T-conformations are highly similar to the X-ray or homology structures. Dark red spheres: N152C-Alexa 647 central atom, yellow: D508C-Alexa 555 central atom (a, c) or S492C-Alexa 555 central atom (b, d). Inset: chemical structures of Alexa dyes modelled as described previously40. The central atoms based on which the distances were measured are labelled. e–h, Close-up of the PPi surrounding residues for A- (e), X- (f–g), and T-conformations (h). A-conformation was modelled by NEST (v1.5)72 based on PDB 3C5E that included the PPi as a part of the ATP and Mg2+. Other conformations were generated by computational modelling in this work. The P-loop (residues 190–199, GrsA numbering) is coloured in orange with the sidechains of T190, G192, T194 and K198 shown in sticks, and the PPi in orange sticks and the Mg2+ as a blue sphere reproduced in (f) and (g) from (e) as reference. The PPi locked in A-conformation by the P-loop can dissociate away in either of two X-conformations, prior to adopting T-conformation. i–j, Inhibition of the high-FRET T-conformation by the PPi in aCC2 (i) and hCC2 (j) shown by bulk-FRET spectra. Fluorescence spectra were normalized by total peak areas. Spectral data are presented as mean ± 1 s.d. from three repeats.
Extended Data Fig. 5 Addition of substrates ATP and L-Phe transiently favours the A-conformation prior to stabilizing the T-conformation in a product-inhibited state.
a, b, Positive correlations between aCC1 and aCC2 (a), and between hCC1 and hCC2 (b) in bulk-FRET steady-state distances under various substrate conditions showing a minimal population of the A-conformation (<10%) that has distinct CC1 and CC2 distances. Bulk-FRET distance errors were propagated from 1 s.d. of three measurements of bulk-FRET transfer efficiency, those from measured extinction coefficients, labelling efficiency and calculated Förster radius \({R}_{\circ }\). c, d, Steady-state bulk-FRET spectra of aCC2 (c) and hCC2 (d) in smFRET buffer, 1-mM ATP, 1-mM L-Phe and 1-mM ATP/L-Phe. Fluorescence spectra were normalized by total peak areas. Spectral data are presented as mean ± 1 s.d. from three repeats. e, Stopped-flow bulk-FRET measurements of aCC2 (150 nM, n = 4 for both substrates and n = 3 for other conditions) revealed a transient biasing of A-conformation after mixing with ATP/L-Phe. A single-exponential fit is shown in black. f, Adenylation-inactive aCC2-K517A (50 nM, n = 4 for both substrates and L-Phe-only and n = 3 for other conditions) shows no changes in the stopped-flow bulk-FRET measurements within 2 s in all four solutions. Data in e and f are shown as mean ± 1 s.d. from specified number of stopped-flow FRET traces. g, Close-up of the modelled active site of the GrsA A-domain in A-conformation (PDB 1AMU with L-Phe). ATP and Mg2+ were taken from PDB 3C5E after aligning the proteins. h, smFRET distance histograms showing that aCC2-K517A is folded and capable of binding Phe-AMS. The solid lines are conformational probability density functions extracted by maximum-entropy deconvolution. The uncertainty was plotted in dash as 1 s.d. from 25 bootstrapped data sets. Inset: a native PAGE gel showing aCC2-K517A and hCC2-K517A binding to Phe-AMS. n = 2 for independently repeated native PAGE assays with similar mobility as shown in the (h) inset. Bottom: smFRET peak positions (uncertainty indicated by translucent vertical bars’ thickness), relative populations with vertical error bars, and normalized transition rates (horizontal arrows). Centres of peak positions and populations are shown as motional narrowing fits of nine time-resolved smFRET conformational distributions and errors are 1 s.d. of motional narrowing fits from 25 bootstrapped smFRET datasets.
Extended Data Fig. 6 X-conformations based on R416 and E362 related inter-subdomain salt bridges are structurally extended.
a–d, Homologous A-domain X-ray structures showing a bent conformation with larger inter-subdomain separation than A-conformation. This conformation is further modelled by MD simulations and shown to feature R416, likely an anchor residue in the AN subdomain. The AN and AC subdomains are coloured in slate and grey, respectively. e–f, Sequence conservation shows that R416 is highly conserved (e) while E362 is marginally conserved (f). Profiles were generated by WebLogo (v2.8.2)71. g–h, Potential salt-bridges were identified between R416 and E444/E441 by smFRET-guided MD simulations for A-domains of aCC1 (g) and aCC2 (h). Inset: close-up of the salt bridge between R416 in the AN subdomain (slate) and E444 (g) or E441 (h) in the AC subdomain (grey), zoomed in from black boxes in the full views in (g) and (h). i–j, Potential salt-bridges were identified between E362 and K434/R439 by smFRET-guided MD simulations for A-domains of aCC1 (i) and aCC2 (j). Inset: close-up of the salt bridge between E362 and K434 or R439. Dark red spheres: N152C-Alexa 647 central atom, yellow spheres: D508C-Alexa 555 central atom (g, i) or S492C-Alexa 555 central atom (h, j). Dyes in (g–j) are shown in sticks.
Extended Data Fig. 7 Coupled intra- and interdomain conformational transitions in GrsA A-PCP didomain.
a, b, Steady-state intra-A-domain distances (dependent on the plateau values) couple with the substrate concentrations, that is, the chemical equilibrium positions. (a), Time-dependent increases of FRET-sensitized acceptor emission with the ATP concentration fixed at 1-mM but varied L-Phe concentrations from top to bottom: 1000 µM ((6.13 ± 0.21)×10−3 s−1), 100 µM ((5.93 ± 0.20)×10−3 s−1), 5 µM ((6.33 ± 0.30)×10−3 s−1), 1 µM ((7.93 ± 0.53)×10−3 s−1), and 0.1 µM ((10.2 ± 1.6)×10−3 s−1). (b), Similar measurements with L-Phe concentration fixed at 1-mM but varied ATP concentrations from top to bottom: 1000 µM ((6.13 ± 0.21)×10−3 s−1), 100 µM ((6.47 ± 0.26)×10−3 s−1), 5 µM ((6.24 ± 0.24)×10−3 s−1), 1 µM ((6.33 ± 0.27)×10−3 s−1), and 0.1 µM ((7.05 ± 0.63)×10−3 s−1). Solid lines represent the single-exponential fits for which the fitting results were given in the parentheses above. In (a–b), errors were plotted as mean ± 1 s.d. from three bulk-FRET kinetic runs. c–d, Similar conformational relaxation rates measured by different probes with mM-substrate concentrations (c for apo constructs and d for holo construct). Comparison of bulk-FRET kinetics for CC1 (n = 3 for aCC1 and hCC1 with 1 mM ATP/L-Phe), CC2 (n = 6 for aCC2 and n = 9 for hCC2 with 1-mM ATP/L-Phe) and sensor 1: A(Alexa546 labelled at N152C)-PCP-EGFP sensor15 (n = 2 for apo 1 with 1 mM ATP/L-Phe and n = 3 for holo 1 with 2 mM ATP/L-Phe where the holo 1 data were replotted from ref. 15) shows the rate similarity between these three constructs (rates are shown in the inset). Data are presented as mean ± 1 s.d. from the specified number of bulk-FRET kinetic runs. e, A-, T-, R416 X- and E362 X-conformations show distinct C-terminal orientations of A-domain. The conserved LPxP motif is coloured as red Cα spheres. The A-conformation is from PDB 1AMU and other conformations are from MD simulations. Close-up: maximal distances between the P526 Cα (red spheres) from any two of the four A-domain structures are 24 Å between T- and E362 X-conformation and 23 Å between A- and E362 X-conformation. f, Structural comparison between the GrsA A-domain in R416 X-state (top) and the LgrA in the formylation state (PDB 5ES9 chain A, bottom), showing the orientation of the AC subdomain is similar in the two structures. It suggests possible roles of the X-conformations in facilitating substrate processing post thiolation. g, Front, top and back views of T-, R416 X- and E362 X-conformations with residues 298–309, 323–328 and 426–444 coloured in light orange. These three stretches of GrsA have been shown with enhanced hydrogen–deuterium exchange rates with 1 mM ATP, 1 mM L-Phe and 5 mM PPi than with 1 mM ATP and 1 mM L-Phe16. h, Overlaid homologous A-PCP didomain structures with the A-domain in A-, T- and R416 X-conformations. The X-ray structures from LgrA were used as template (see PDB ID’s in the text inset). T- and A-conformations of the A-PCP didomain were modelled using Phyre2 (v2.0)58 and R416 X-conformation of the didomain was modelled by NEST (v1.5)72. The labelled PCP-to-PCP distance was measured from S573 Cα (cyan spheres) and the AC-to-AC distance was measured from P526 Cα. In all cases, the PCP travels greater distances than the C-terminus of the AC subdomain.
Extended Data Fig. 8 R416E is a folded GrsA mutants with a low thioester formation activity on the hour timescale.
a. FRET spectra of dye-labelled aCC2-R416E with and without urea. Fluorescence spectra were normalized by total peak areas. Spectral data are presented as mean ± 1 s.d. from three repeats. b–d, CD spectra of aWT (b), aCC2-R439A (c) and aCC2-R416E (d) with and without 4 M urea. The mean residue ellipticity (MRE) data are shown as mean ± 1 s.d. from three repeats. Due to the 300 mM NaCl used in smFRET buffer as well as 4 M urea in the denaturation experiments, the minimal wavelength with reliable dynode voltages (around 800 V) was 215 nm, below which the CD signals became unreliable. Because the overall CD signals followed a similar trend for all the three mutants, the amplitude difference in MRE was mostly due to variations in concentration (~10%). e. ESI-TOF MS spectra showing that hCC2-R416E is biochemically active on the hour timescale with multiple Phe-adducts formed. f, T-state populations (T%) with and without substrates for hCC2, hCC2-E362K and hCC2-R416E. g–h, the millisecond X/T exchange rates from smFRET experiments without (g) and with 1 mM ATP/L-Phe (h). Centres of error bars in (f–h) are shown as motional narrowing fits of nine time-resolved smFRET conformational distributions and errors are 1 s.d. of motional narrowing fits from 25 bootstrapped smFRET datasets.
Extended Data Fig. 9 aCC2 SEC-SAXS datasets with or without substrates.
a, b, Rg values determined by Guinier analysis, plotted across the elution peak (a, substrate-free) and (b, 1 mM ATP/L-Phe). The left shoulders of the elution peaks with elevated Rg values indicate minor populations of aggregates, whereas the Rg stays constant between frames ~500 and ~550 (a) and frames ~850 and ~900 (b). c, d, The elution in both cases was separated cleanly into three components by evolving factor analysis (EFA)28,68: aggregate (top), monomeric protein (middle), and mismatched buffer component (bottom). The EFA-separated elution peak for each component is highlighted in grey, and the corresponding EFA-separated SAXS profile is shown to the right (c, substrate-free and d, incubated with 1 mM ATP/L-Phe). The intensity data are presented as mean ± 1 s.e.m., where errors estimates were propagated from photon counting errors as described in ref. 28. e, f, Guinier fit to the low-q region of the EFA-separated monomeric protein component (e, substrate-free and f, incubated with 1 mM ATP/L-Phe).
Extended Data Fig. 10 SAXS analysis shows significant compaction of the didomain aCC2 construct upon addition of substrates.
a, EFA-separated SAXS profiles of aCC2 (black) and aCC2 incubated with 1 mM ATP/L-Phe (blue), shown as a semilog plot, display a clear change in shape upon the addition of 1 mM ATP/L-Phe, indicative of a change in protein conformation. The intensity was normalized by I(0) for comparison. b, Plotting the scattering profiles in Kratky representation emphasizes the change in the mid-q region (~0.1–0.2 Å−1), which is sensitive to domain arrangements. The addition of substrates leads to a tightening in the shape of the Kratky peak, consistent with a significant compaction. c, d, Comparison of EOM fits done by defining 2 rigid bodies (A-domain, residues 17–527, and PCP, 537–614) to EOM fits done by defining 3 rigid bodies (AN subdomain, 17–427, AC subdomain, 432–527, and PCP, 537–614). (c), Fits to the aCC2 scattering in the absence of substrate. The two fits overlay well; the quality of the 2-rigid body fit (solid curve, χ2 = 1.98) is comparable to the 3-rigid body fit (dashed curve, χ2 = 1.71). (d), Fits to the aCC2 with 1 mM ATP/L-Phe scattering. The two fits again overlay well; the quality of the 2-rigid body fit (solid curve, χ2 = 2.81) is comparable to the 3-rigid body fit (dashed curve, χ2 = 2.63). As adding an additional degree of freedom in the 3-rigid bodies analysis does not significantly improve the quality of the fits from the 2-rigid bodies analysis, our SEC-SAXS data provide independent experimental support to our smFRET-guided MD modelling (Extended Data Fig. 1).
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Notes 1 and 2, Discussion and references.
Supplementary Data 1
smFRET results for all mutants used in this work with various solution conditions.
Supplementary Data 2
Primer designs for site-directed mutagenesis.
Supplementary Video 1
Unconstrained MD simulations of A-conformation for the aCC1 construct. The AN and AC subdomains are coloured in slate and grey, respectively. The CC1-dye models were included with the donor in yellow and the acceptor in red. Each frame of the movie was taken from a 16 ps time step from the MD simulations and the time course of the entire movie is 4 ns.
Supplementary Video 2
MD simulations of X-conformations with salt bridges between R416 and E444/E441. The AN and AC subdomains are coloured in slate and grey, respectively. Visualization of an all-atom smFRET-distance-constrained MD simulation shows dynamic R416–E444 and R416–E441 interactions coloured in purple. The E362–K434 salt bridge was coloured in magenta. The salt-bridge stick representations are dull when minimal side-chain N–O bond distances among participating residues were greater than 4 Å. The CC2-dye models were included with the donor in yellow and the acceptor in red. Each frame of the movie was taken from a 16 ps time step from the MD simulation and the time course of the entire movie is 4 ns.
Supplementary Video 3
MD simulations of extended A-domain conformations. Visualization of a typical self-guided Langevin dynamics simulation started from the A-conformation and rapidly transitioned to extended conformations. The AN and AC subdomains are coloured in slate and grey, respectively. The salt-bridge between R416 and E444 (purple) in the A-conformation broke in the first frame while that of E362 and K434 (magenta) intermittently formed later. The salt-bridge stick representations are dull when the side-chain N–O bond distances were greater than 4 Å. The time course of the entire movie is 4 ns.
Supplementary Video 4
MD simulations of X-conformations with salt bridges between E362 and K434/R439. The AN and AC subdomains are coloured in slate and grey, respectively. Visualization of an all-atom smFRET-distance-constrained MD simulation shows dynamic salt-bridge interactions between E362 and K434/R439 (magenta). The FRET-dye models were included at the CC2 labelling points with the donor and acceptor dyes coloured yellow and red, respectively. The salt-bridge stick representations are dull when minimal side-chain N–O bond distances among participating residues were greater than 4 Å. Each frame of the movie was taken from a 16 ps time step from the MD simulation and the time course of the entire movie is 4 ns.
Source data
Source Data Fig. 1
Statistical and numerical source data for Fig. 1.
Source Data Fig. 2
Statistical and numerical source data for Fig. 2.
Source Data Fig. 3
Statistical and numerical source data for Fig. 3.
Source Data Fig. 4
Numerical source data for Fig. 4.
Source Data Extended Data Fig. 1
Statistical and numerical source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Statistical and numerical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 2
Unprocessed native gel for Extended Data Fig. 2b,c.
Source Data Extended Data Fig. 3
Statistical and numerical source data for Extended Data Fig.3c–l.
Source Data Extended Data Fig. 4
Numerical source data for Extended Data Fig. 4i–j.
Source Data Extended Data Fig. 5
Statistical and numerical source data for Extended Data Fig. 5
Source Data Extended Data Fig. 5
Unprocessed native gel for Extended Data Fig. 5h.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7a–d.
Source Data Extended Data Fig. 8
Statistical and numerical source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Numerical source data for Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Numerical source data for Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Alfermann, J., Li, H. et al. Subdomain dynamics enable chemical chain reactions in non-ribosomal peptide synthetases. Nat. Chem. 16, 259–268 (2024). https://doi.org/10.1038/s41557-023-01361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01361-4