Abstract
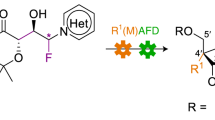
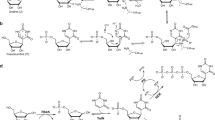
Nucleoside diphosphates and triphosphates impact nearly every aspect of biochemistry; however, the use of such compounds as tools or medicinal leads for nucleotide-dependent enzymes and receptors is hampered by their rapid in vivo metabolism. Although a successful strategy to address the instability of the monophosphate moiety in oligonucleotide therapeutics has been accomplished by their isosteric replacement with phosphorothioates, no practical methods exist to rapidly and controllably access stereopure di- and triphosphate thioisosteres of both natural and unnatural nucleosides. Here we show how a modular, reagent-based platform can enable the stereocontrolled and scalable synthesis of a library of such molecules. This operationally simple approach provides access to pure stereoisomers of nucleoside α-thiodiphosphates and α-thiotriphosphates, as well as symmetrical or unsymmetrical dinucleoside thiodiphosphates and thiotriphosphates (including RNA cap reagents). We demonstrate that ligand–receptor interactions can be dramatically influenced by P-stereochemistry, showing that such thioisosteric replacements can have profound effects on the potency and stability of lead candidates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study (including experimental procedures, optimization details and spectral data for all new compounds) is available within the paper and Supplementary Information.
X-ray crystallographic data for compound (−)-11 have been deposited with the Cambridge Crystallographic Data Centre (CCDC deposition no. 2172688). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Roy, B., Depaix, A., Périguad, C. & Peyrottes, S. Recent trends in nucleotide synthesis. Chem. Rev. 116, 7854–7897 (2016).
Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry 7th edn (W. H. Freeman, 2017).
Vetter, I. R. & Wittinghofer, A. Nucleoside triphosphate-binding proteins: different scaffolds to achieve phosphoryl transfer. Q. Rev. Biophys. 32, 1–56 (1999).
Erb, L. & Weisman, G. A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 789–803 (2012).
Vosberg, H. P. & Eckstein, F. Effect of deoxynucleoside phosphorothioates incorporated in DNA on cleavage by restriction enzymes. J. Biol. Chem. 257, 6595–6599 (1982).
Crooke, S. T. Antisense Research and Application (Springer, 1998).
Wickstrom, E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J. Biochem. Biophys. Methods. 13, 97–102 (1986).
Purcell, J. & Hengge, A. C. The thermodynamics of phosphate versus phosphorothioate ester hydrolysis. J. Org. Chem. 70, 8437–8442 (2005).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Nadel, Y. et al. Highly potent and selective ectonucleotide pyrophosphatase/phosphodiesterase I inhibitors based on an adenosine 5′-(α or γ)-thio-(α,β- or β,γ)-methylenetriphosphate scaffold. J. Med. Chem. 57, 4677–4691 (2014).
Lee, S.-Y. & Müller, C. E. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm 8, 823–840 (2017).
Kato, K. et al. Structural insights into cGAMP degradation by ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat. Commun. 9, 4424 (2018).
Jacobson, K. A. et al. Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5′-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem. Pharmacol. 71, 540–549 (2006).
Kowalska, J. et al. Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS. RNA 14, 1119–1131 (2008).
Wojtczak, B. A. et al. 5′-Phosphorothiolate dinucleotide cap analogues: reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. J. Am. Chem. Soc. 140, 5987–5999 (2018).
Yang, Z., Sismour, A. M. & Benner, S. A. Nucleoside α-thiotriphospates, polymerases and the exonuclease III analysis of oligonucleotides containing phosphorothiote linkages. Nucleic Acids Res. 35, 3118–3127 (2007).
McIntosh, J. A. et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature 603, 439–444 (2022).
Ludwig, J. & Eckstein, F. Synthesis of nucleoside 5′-O-(1,3-dithiotriphosphates) and 5′-O-(1,1-dithiotriphosphates). J. Org. Chem. 56, 1777–1783 (1991).
Strenkowska, M., Wanat, P., Ziemniak, M., Jemielity, J. & Kowalska, J. Preparation of synthetically challenging nucleotides using cyanoethyl P-imidazolides and microwaves. Org. Lett. 14, 4782–4785 (2012).
Misiura, K., Szymanowicz, D. & Stec, W. J. Synthesis of nucleoside α-thiotriphosphates via an oxathiophospholane approach. Org. Lett. 7, 2217–2220 (2005).
Li, P. et al. Synthesis of α-P-modified nucleoside diphopshates with ethylenediamine. J. Am. Chem. Soc. 127, 16782–16783 (2005).
Connolly, B. A., Romaniuk, P. J. & Eckstein, F. Synthesis and characterization of diastereoisomers of guanosine 5′-O-(1-thiotriphosphate) and 5′-O-(2-thiotriphosphate). Biochemistry 21, 1983–1989 (1982).
Eckstein, F. & Gindl, H. Synthesis of nucleoside 5′-polyphosphorothioates. Biochim. Biophys. Acta 149, 35–40 (1967).
Guga, P. & Tomaszewska, A. Unexpected loss of stereoselectivity in ring-opening reaction of 2-alkoxy-2-thio-1,3,2-oxathiophospholanes with a pyrophosphate anion. Chirality 27, 115–122 (2015).
Dahnke, T., Jiang, R. T. & Tsai, M. D. Mechanism of adenylate kinase. 12. Prediction and demonstration of enhancement of phosphorus stereospecifity by site-directed mutagenesis. J. Am. Chem. Soc. 113, 9388–9389 (1991).
Knouse, K. et al. Unlocking P(V): reagents for chiral phosphorothioate synthesis. Science 361, 1234–1238 (2018).
Huang, Y. et al. A P(V) platform for oligonucleotide synthesis. Science 373, 1265–1270 (2021).
Knouse, K. W. et al. Nature chose phosphates and chemists should too: how emerging P(V) methods can augment existing strategies. ACS Cent. Sci. 7, 1473–1485 (2021).
Zheng, B. et al. P(III) vs P(V): a P(V) reagent for thiophosphoramidate linkages and application to an asymmetric synthesis of a cyclic dinucleotide STING agonist. J. Org. Chem. 87, 1934–1940 (2022).
Liao, J.-Y., Bala, S., Ngor, A. K., Yik, E. J. & Chaput, J. C. P(V) reagents for the scalable synthesis of natural and modified nucleoside triphosphates. J. Am. Chem. Soc. 141, 13286–13289 (2019).
Cremosnik, G. S., Hofer, A. & Jessen, H. J. Iterative synthesis of nucleoside oligophosphates with phosphoroamidites. Angew. Chem. Int. Ed. 53, 286–289 (2014).
Ociepa, M. et al. Mild and chemoselective phosphorylation of alcohols using a Ψ-reagent. Org. Lett. 23, 9337–9342 (2021).
Ludwig, J. & Eckstein, F. Rapid and efficient synthesis of nucleoside 5′-O-(1-thiotriphosphates), 5′-triphosphates and 2′,3′-cyclophosphorothioates using 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one. J. Org. Chem. 54, 631–635 (1989).
Masawra, A. et al. Synthesis and stereochemical assignment of crypto-optically active 2H6-neopentane. Angew. Chem. Int. Ed. 54, 13106–13109 (2015).
Roucairol, C. et al. Design, synthesis and studies of triphosphate analogues for the production of anti AZT-TP antibodies. Bioorg. Med. Chem. Lett. 20, 987–990 (2010).
Cusack, N. J. & Hourani, S. M. O. Effects of RP and SP diastereoisomers of adenosine 5′-O-(1-thiodiphosphate) on human platelets. Br. J. Pharmacol. 73, 409–412 (1981).
Johnson, R. A., Desaubry, L. & Shoshani, I. Method and compound for the inhibition of adenylyl cyclase. US patent US5795756A (1998).
Ecke, D., Fischer, B. & Reiser, G. Diastereoselectivity of the P2Y11 nucleotide receptor: mutational analysis. Br. J. Pharmacol. 155, 1250–1255 (2008).
Besada, P. et al. Structure-activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J. Med. Chem. 49, 5532–5543 (2006).
Shuman, S. DNA ligases: progress and prospects. J. Biol. Chem. 284, 17365–17369 (2009).
Péregrin, P. Purinergic Signaling. Methods in Molecular Biology, Vol. 2041 (Humana, 2019).
Yegutkin, G. G. & Boison, D. ATP and adenosine metabolism in cancer: exploitation of therapeutic gain. Pharmacol. Rev. 74, 797–822 (2022).
Kiselev, E. et al. Exploring a 2-naphthoic acid template for the structure-based design of P2Y14 receptor antagonist molecular probes. ACS Chem. Biol. 9, 2833–2842 (2014).
Hyjek-Składanowska, M. et al. Origins of the increased affinity of phosphorothioate-modified therapeutic nucleic acids for proteins. J. Am. Chem. Soc. 142, 7456–7468 (2020).
Schäkel, L. et al. Protein kinase inhibitor ceritinib blocks ectonucleotidase CD39—a promising target for cancer immunotherapy. J. Immunother. Cancer 10, e004660 (2022).
Zhang, B. CD73: a novel target for cancer immunotherapy. Cancer Res. 70, 6407–6411 (2010).
Kuhn, A. N. et al. Phosphorothiate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 17, 961–971 (2010).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Romagnoli, A. et al. Control of the eIF4E activity: structural insights and pharmacological implications. Cell. Mol. Life Sci. 78, 6869–6885 (2021).
van Dijk, E. et al. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 21, 6915–6924 (2002).
Acknowledgements
We are grateful to D.-H. Huang and L. Pasternack (Scripps Research) for NMR spectroscopic assistance, B. Sanchez, Q.N. Wong and J. Chen for HRMS assistance, M. Gembicky for X-ray crystallographic analysis, A. Bauer, M. Meanwell, M. Bielecki, A.F. Garrido Castro, C. He, Y. Kawamata and S. Gnaim for insightful discussions, and T.E.-H. Ewing for analytical assistance. Financial support for this work was provided by Bristol-Myers Squibb. M.O. was supported by the Polish National Agency for Academic Exchange (Bekker programme no. PPN/BEK/2020/1/00111/U/00001). H.-J.Z. was supported by Shanghai Institute of Organic Chemistry Fellowship. M.N. thanks the Council for Higher Education, Fulbright Israel. K.A.J thanks the NIH, NIDDK, for funding (ZIADK031116). C.E.M. and co-workers were funded by the Deutsche Forschungsgemeinschaft (SFB 1328). J.J. and co-workers were supported by the Polish National Science Centre (grant no. 2019/33/B/ST4/01843). Bristol Myers Squibb assisted with the conceptualization of this work. The other funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
H.-J.Z., M.O., M.N., M.A.S., M.D.E. and P.S.B. conceptualized the study. H.-J.Z., M.O., M.N. and B.Z. developed the reagents. H.-J.Z., M.O. and M.N. conducted optimization and scope experiments. H.-J.Z., M.O., M.N. and Z.L. analysed the data. H.B., J.N., S.M., H.A.-H., B.B. and C.E.M. designed and performed biological experiments on the P2Y13 and P2X receptors and ecto-enzymes. S.A.L., V.S. and K.A.J. designed and performed biological evaluation on the P2Y6 and P2Y14 receptors. V.S. and K.A.J. performed molecular modelling. O.P., J.K. and J.J. designed and performed CleanCap thioisosteres biophysical studies. H.-J.Z., M.O., M.N., C.E.M., K.A.J., J.K., J.J. and P.S.B. wrote the manuscript. P.S.B., M.A.S. and M.D.E. acquired funding. P.S.B. administered and supervised this work.
Corresponding authors
Ethics declarations
Competing interests
A provisional US patent application on this work has been filed by Bristol-Myers Squibb (application no. 63/376,249), with H.-J.Z., M.O., M.N., B.Z., M.A.S., M.D.E. and P.S.B. listed as inventors. The patent covers the synthesis of new reagents and their applications to the synthesis of nucleotide thioisosteres. P.S.B. is a paid consultant for Bristol Myers Squibb. B.Z., Z.L., M.A.S. and M.D.E. are employees of Bristol Myers Squibb.
Peer review
Peer review information
Nature Chemistry thanks Zlatko Janeba and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and methods, Supplementary Figs. 1–47, Tables 1–18 and spectral data for all new compounds.
Supplementary Data
Crystallographic data for compound (−)-11; CCDC reference 2172688.
Source data
Source Data Fig. 3
Unprocessed numerical data for graphs 3c, 3d, 3g, 3h, 3i, 3j.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, HJ., Ociepa, M., Nassir, M. et al. Stereocontrolled access to thioisosteres of nucleoside di- and triphosphates. Nat. Chem. 16, 249–258 (2024). https://doi.org/10.1038/s41557-023-01347-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01347-2