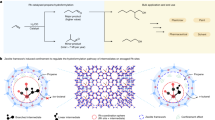
Abstract
Despite recent achievements in the field of frustrated Lewis pairs (FLPs) for small molecule activations, the reversible activation and catalytic transformations of N–H-activated ammonia remain a challenge. Here we report on a rare combination of an aluminium Lewis acid and a carbon Lewis base. A so-called hidden FLP consisting of a phosphorus ylide featuring an aluminium fragment in the ortho position of a phenyl ring scaffold is introduced. Although the formation of the Lewis acid/base adduct is observed in the solid state, which at first glance leads to formally quenched FLP reactivity, we show that the title compound readily reacts with non-aqueous ammonia thermoneutrally and splits the N–H bond reversibly at ambient temperature. In addition, NH3 transfer reactions mediated by a main-group catalyst are presented. This proof-of-principle study is expected to initiate further activities in utilizing N–H-activated ammonia as a readily available, atom-economical nitrogen source.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. Raw and unprocessed NMR, HRMS and gel permeation chromatography data are available from figshare (https://doi.org/10.6084/m9.figshare.24042771)54. Materials and methods, computational studies including cartesian coordinates and energies for the computed structures, experimental procedures, characterization data, NMR spectra and mass spectrometry data are available in the paper with further details in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2180947 (2) and 2180948 (3). For further crystallographic details see Supplementary Section 2. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Streiff, S. & Jérôme, F. Hydroamination of non-activated alkenes with ammonia: a holy grail in catalysis. Chem. Soc. Rev. 50, 1512–1521 (2021).
Werner, A. Coordination chemistry. Z. Anorg. Chem. 3, 267–330 (1893).
Bezdek, M. J., Guo, S. & Chirik, P. J. Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex. Science 354, 730–733 (2016).
Bryan, E. G., Johnson, B. F. G. & Lewis, J. 1,1,2,2,2,2,3,3,3,3-Decacarbonyl-1-(η-cyclohexa-1,3-diene)-triangulo-triosmium: a novel intermediate in synthetic osmium cluster chemistry. J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/DT9770001328 (1977).
Hillhouse, G. L. & Bercaw, J. E. Reactions of water and ammonia with bis (pentamethylcyclopentadienyl) complexes of zirconium and hafnium. J. Am. Chem. Soc. 106, 5472–5478 (1984).
Casalnuovo, A. L., Calabrese, J. C. & Milstein, D. Rational design in homogeneous catalysis. Iridium(I)-catalyzed addition of aniline to norbornylene via nitrogen-hydrogen activation. J. Am. Chem. Soc. 110, 6738–6744 (1988).
Holl, M. M. B., Wolczanski, P. T. & Van Duyne, G. D. The ladder structure of [(tert-BuCH2)2TaN]5·NH3·2C7H8 and its relationship to cubic tantalum nitride. J. Am. Chem. Soc. 112, 7989–7994 (1990).
Braun, T. Oxidative addition of NH3 to a transition-metal complex: a key step for the metal-mediated derivatization of ammonia? Angew. Chem. Int. Ed. 44, 5012–5014 (2005).
Zhao, J., Goldman, A. S. & Hartwig, J. F. Oxidative addition of ammonia to form a stable monomeric amido hydride complex. Science 307, 1080–1082 (2005).
Nakajima, Y., Kameo, H. & Suzuki, H. Cleavage of nitrogen–hydrogen bonds of ammonia induced by triruthenium polyhydrido clusters. Angew. Chem. Int. Ed. 45, 950–952 (2006).
Morgan, E., MacLean, D. F., McDonald, R. & Turculet, L. Rhodium and iridium amido complexes supported by silyl pincer ligation: ammonia N−H bond activation by a [PSiP]Ir complex. J. Am. Chem. Soc. 131, 14234–14236 (2009).
Salomon, M. A., Jungton, A.-K. & Braun, T. Activation of ethylene and ammonia at iridium: C–H versus N–H oxidative addition. J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/B906189D (2009).
Ni, C., Lei, H. & Power, P. P. Reaction of M(II) diaryls (M = Mn or Fe) with ammonia to afford parent amido complexes. Organometallics 29, 1988–1991 (2010).
Brown, R. M. et al. Ammonia activation by a nickel NCN–pincer complex featuring a non-innocent N-heterocyclic carbene: ammine and amido complexes in equilibrium. Angew. Chem. Int. Ed. 54, 6274–6277 (2015).
Scheibel, M. G. et al. Homolytic N–H activation of ammonia: hydrogen transfer of parent iridium ammine, amide, imide, and nitride species. Inorg. Chem. 54, 9290–9302 (2015).
Margulieux, G. W., Bezdek, M. J., Turner, Z. R. & Chirik, P. J. Ammonia activation, H2 evolution and nitride formation from a molybdenum complex with a chemically and redox noninnocent ligand. J. Am. Chem. Soc. 139, 6110–6113 (2017).
LaPierre, E. A., Piers, W. E. & Gendy, C. Redox-state dependent activation of silanes and ammonia with reverse polarity (PCcarbeneP)Ni complexes: electrophilic vs. nucleophilic carbenes. J. Chem. Soc. Dalton Trans. 47, 16789–16797 (2018).
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Weetman, C. & Inoue, S. The road travelled: after main-group elements as transition metals. ChemCatChem 10, 4213–4228 (2018).
Hansmann, M. M. & Bertrand, G. Transition-metal-like behavior of main group elements: ligand exchange at a phosphinidene. J. Am. Chem. Soc. 138, 15885–15888 (2016).
Wang, Y. & Liu, C.-G. The use of main-group elements to mimic catalytic behavior of transition metals I: reduction of dinitrogen to ammonia catalyzed by bis(Lewis base)borylenium diradicals. Phys. Chem. Chem. Phys. 22, 28423–28433 (2020).
Frey, G. D., Lavallo, V., Donnadieu, B., Schoeller, W. W. & Bertrand, G. Facile splitting of hydrogen and ammonia by nucleophilic activation at a single carbon center. Science 316, 439–441 (2007).
Wang, Y. et al. Activation of ammonia by a carbene-stabilized dithiolene zwitterion. J. Am. Chem. Soc. 144, 16325–16331 (2022).
Yang, X. et al. Radical activation of N–H and O–H bonds at bismuth(II). J. Am. Chem. Soc. 144, 16535–16544 (2022).
Abbenseth, J., Townrow, O. P. E. & Goicoechea, J. M. Thermoneutral N−H bond activation of ammonia by a geometrically constrained phosphine. Angew. Chem. Int. Ed. 60, 23625–23629 (2021).
Welch, G. C., Juan, R. R. S., Masuda, J. D. & Stephan, D. W. Reversible, metal-free hydrogen activation. Science 314, 1124–1126 (2006).
Stephan, D. W. & Erker, G. Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew. Chem. Int. Ed. 49, 46–76 (2010).
Stephan, D. W. Frustrated Lewis pairs. J. Am. Chem. Soc. 137, 10018–10032 (2015).
Stephan, D. W. Frustrated Lewis pairs: from concept to catalysis. Acc. Chem. Res. 48, 306–316 (2015).
Stephan, D. W. & Erker, G. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Ed. 54, 6400–6441 (2015).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Mahdi, T. & Stephan, D. W. Frustrated Lewis pair catalyzed hydroamination of terminal alkynes. Angew. Chem. Int. Ed. 52, 12418–12421 (2013).
Mahdi, T. & Stephan, D. W. Stoichiometric and catalytic inter- and intramolecular hydroamination of terminal alkynes by frustrated Lewis pairs. Chem. Eur. J. 21, 11134–11142 (2015).
Ma, G., Song, G. & Li, Z. H. Designing metal-free frustrated Lewis pairs catalyst for the efficient dehydrogenation of ammonia borane. Chem. Eur. J. 24, 13238–13245 (2018).
Zhang, L. et al. Catalytic dehydrogenation of ammonia borane mediated by a Pt(0)/borane frustrated Lewis pair: theoretical design. ChemPhysChem 21, 2573–2578 (2020).
Sarbajna, A., Swamy, V. S. V. S. N. & Gessner, V. H. Phosphorus-ylides: powerful substituents for the stabilization of reactive main group compounds. Chem. Sci. 12, 2016–2024 (2021).
Krämer, F., Radius, M., Hinz, A., Dilanas, M. E. A. & Breher, F. Accessing cationic α-silylated and α-germylated phosphorus ylides. Chem. Eur. J. 28, e202103974 (2022).
Schier, A. & Schmidbaur, H. Derivatives and coordination compounds of triphenylphosphonium cyclopropylide (C6H5)3P=C(CH2)2. Z. Naturforsch. B 37, 1518–1523 (1982).
Dureen, M. A. & Stephan, D. W. Biphenylamide ligand complexes of Li and Al: hemilabile arene donors? J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/B801772G (2008).
Swarnakar, A. K. et al. Application of the donor–acceptor concept to intercept low oxidation state group 14 element hydrides using a Wittig reagent as a Lewis base. Inorg. Chem. 53, 8662–8671 (2014).
Korth, K. & Sundermeyer, J. Interaction of t-butyllithium and triphenylmethylenephosphoranes. Tetrahedron Lett. 41, 5461–5464 (2000).
Radius, M. & Breher, F. α-Borylated phosphorus ylides (α-BCPs): electronic frustration within a C−B π-bond arising from the competition for a lone pair of electrons. Chem. Eur. J. 24, 15744–15749 (2018).
Wiberg, N. Lehrbuch der Anorganischen Chemie. 102nd edn (W. de Gruyter, 2007).
Brook, P. R. & Duke, A. J. Ring-opening reactions of 7,7-dichlorobicyclo[3,2,0]hept-2-en-6-one and its conversion into methyl benzoate with methoxide ion. J. Chem. Soc. C https://doi.org/10.1039/J39710001764 (1971).
Roedig, A., Bonse, G., Ganns, E. M. & Heinze, H. Der einfluss der perchlorsubstitution auf einige ringöffnungsreaktionen des benzocyclobutendions. Tetrahedron 33, 2437–2440 (1977).
Argouarch, G., Stones, G., Gibson, C. L., Kennedy, A. R. & Sherrington, D. C. Bifurcated, modular syntheses of chiral annulet triazacyclononanes. Org. Biomol. Chem. 1, 4408–4417 (2003).
Zhang, L. et al. Iridium-catalyzed asymmetric hydrogenation of simple ketones with tridentate PNN ligands bearing unsymmetrical vicinal diamines. J. Org. Chem. 88, 2942–2951 (2023).
Kim, J., Kim, H. J. & Chang, S. Synthetic uses of ammonia in transition-metal catalysis. Eur. J. Org. Chem. 2013, 3201–3213 (2013).
Lawrence, S. A. Amines: Synthesis, Properties and Applications (Cambridge Univ. Press, 2005).
Legnani, L., Bhawal, B. N. & Morandi, B. Recent developments in the direct synthesis of unprotected primary amines. Synthesis 49, 776–789 (2017).
Tshepelevitsh, S. et al. On the basicity of organic bases in different media. Eur. J. Org. Chem. 2019, 6735–6748 (2019).
Uhl, W., Wagner, J., Fenske, D. & Baum, G. N,N,N′,N′-Tetramethylethylendiamin-di(tert-butyl)aluminium-Kationen—Molekülstruktur des [(Me3C)2Al · TMEDA]⊕[(Me3C)2AlBr2]⊖ Z. Anorg. Allg. Chem. 612, 25–34 (1992).
Allen, C. L., Burel, C. & Williams, J. M. J. Cost efficient synthesis of amides from oximes with indium or zinc catalysts. Tetrahedron Lett. 51, 2724–2726 (2010).
Krämer, F., Paradies, J., Fernández, I. & Breher, F. A crystalline aluminum–carbon-based ambiphile capable of activation and catalytic transfer of ammonia. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2022-d37lg (2022).
Acknowledgements
This work was partly carried out with the support of the Karlsruhe Nano Micro Facility, a Helmholtz research platform at KIT, and we thank D. Fenske, A. Hinz and B. Birenheide for help with X-ray diffraction. We also thank K. Kohnle, A. Mösle, L. Hirsch and A. Hochgesand from the Institute of Organic Chemistry at KIT for performing mass spectral and elemental analysis. R. Nickisch and L. Santos Correa from the Institute of Organic Chemistry at KIT are acknowledged for their help with gel permeation chromatography measurements. The authors acknowledge support by the state of Baden-Württemberg through bwHPC and the German Research Foundation through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster). I.F. is grateful to the Spanish MCIN/AEI/10.13039/501100011033 (grants PID2019-106184GB-I00 and PID2022-139318NB-I00). The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
F.B. supervised the project. I.F. performed the quantum chemical calculations. J.P. supported the catalytic studies and the van’t Hoff analysis. F.K. conducted all experiments and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Chemistry thanks Cameron Jones and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, discussion and coordinates of the calculated structures.
Supplementary Data 1
Source data for Supplementary Fig. 23a.
Supplementary Data 2
Source data for Supplementary Fig. 23c.
Supplementary Data 3
Crystallographic structure factor data for compound 2; CCDC reference 2180947.
Supplementary Data 4
Crystallographic structure factor data for compound 3; CCDC reference 2180948.
Source data
Source Data Fig. 2
Raw NMR data.
Source Data Fig. 4
Source data for the kinetic studies of the catalytic alkylation of benzylbromide.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krämer, F., Paradies, J., Fernández, I. et al. A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media. Nat. Chem. 16, 63–69 (2024). https://doi.org/10.1038/s41557-023-01340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01340-9