Abstract
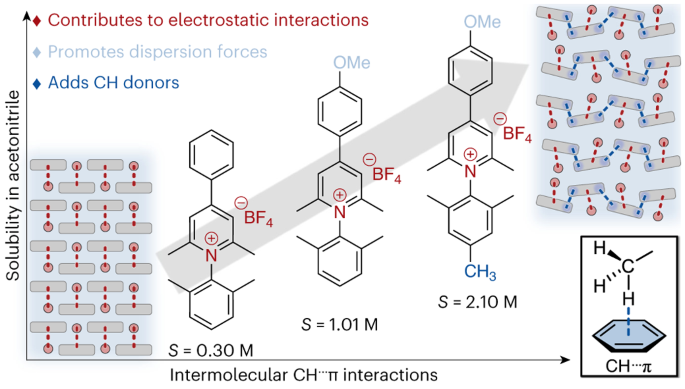
Grid-scale energy storage applications, such as redox flow batteries, rely on the solubility of redox-active organic molecules. Although redox-active pyridiniums exhibit exceptional persistence in multiple redox states at low potentials (desirable properties for energy storage applications), their solubility in non-aqueous media remains low, and few practical molecular design strategies exist to improve solubility. Here we convey the extent to which discrete, attractive interactions between C–H groups and π electrons of an aromatic ring (C–H···π interactions) can describe the solubility of N-substituted pyridinium salts in a non-aqueous solvent. We find a direct correlation between the number of C–H···π interactions for each pyridinium salt and its solubility in acetonitrile. The correlation presented in this work highlights a consequence of disrupting strong electrostatic interactions with weak dispersion interactions, showing how minimal structural change can dramatically impact pyridinium solubility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supporting information files) with the exception of raw voltammetry data and raw spectrophotometry data, which can be provided upon request. Metrical parameters for crystal structures are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC-2162134, 2163981, 2165226, 2157789, 2155765, 2155982, 2162102, 2156178, 2163473, 2155992, 2155704, 2156211, 2156206, 2155715, 2167771, 2157809, 2166708, 2168227, 2157813, 2156563, 2156214 and 2158012. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Winsberg, J., Hagemann, T., Janoschka, T., Hager, M. D. & Schubert, U. S. Redox-flow batteries: from metals to organic redox-active materials. Angew. Chem. Int. Ed. 56, 686–711 (2017).
Li, M., Rhodes, Z., Cabrera-Pardo, J. R. & Minteer, S. D. Recent advancements in rational design of non-aqueous organic redox flow batteries. Sustain. Energy Fuels 4, 4370–4389 (2020).
Luo, J., Hu, B., Hu, M., Zhao, Y. & Liu, T. L. Status and prospects of organic redox flow batteries toward sustainable energy storage. ACS Energy Lett. 4, 2220–2240 (2019).
Kowalski, J. A., Su, L., Milshtein, J. D. & Brushett, F. R. Recent advances in molecular engineering of redox active organic molecules for nonaqueous flow batteries. Curr. Opin. Chem. Eng. 13, 45–52 (2016).
Soloveichik, G. L. Flow batteries: current status and trends. Chem. Rev. 115, 11533–11558 (2015).
Wedege, K., Dražević, E., Konya, D. & Bentien, A. Organic redox species in aqueous flow batteries: redox potentials, chemical stability and solubility. Sci. Rep. 6, 1–13 (2016).
Er, S., Suh, C., Marshak, M. P. & Aspuru-Guzik, A. Computational design of molecules for an all-quinone redox flow battery. Chem. Sci. 6, 885–893 (2015).
Sevov, C. S. et al. Evolutionary design of low molecular weight organic anolyte materials for applications in nonaqueous redox flow batteries. J. Am. Chem. Soc. 137, 14465–14472 (2015).
Robinson, S. G., Yan, Y., Hendriks, K. H., Sanford, M. S. & Sigman, M. S. Developing a predictive solubility model for monomeric and oligomeric cyclopropenium-based flow battery catholytes. J. Am. Chem. Soc. 141, 10171–10176 (2019).
Sevov, C. S. et al. Physical organic approach to persistent, cyclable, low-potential electrolytes for flow battery applications. J. Am. Chem. Soc. 139, 2924–2927 (2017).
Reichardt, C. & Welton, T. Solvents and Solvent Effects in Organic Chemistry (Wiley, 2011).
Hansen, C. M. Hansen Solubility Parameters: A User’s Handbook (CRC Press, 2000).
Barton, A. F. M. Solubility parameters. Chem. Rev. 75, 731–753 (1975).
Geysens, P., Evers, J., Dehaen, W., Fransaer, J. & Binnemans, K. Enhancing the solubility of 1,4-diaminoanthraquinones in electrolytes for organic redox flow batteries through molecular modification. RSC Adv. 10, 39601–39610 (2020).
Attanayake, N. H. et al. Tailoring two-electron-donating phenothiazines to enable high-concentration redox electrolytes for use in nonaqueous redox flow batteries. Chem. Mater. 31, 4353–4363 (2019).
Milshtein, J. D. et al. High current density, long duration cycling of soluble organic active species for non-aqueous redox flow batteries. Energy Environ. Sci. 9, 3531–3543 (2016).
Huang, J. et al. Liquid catholyte molecules for nonaqueous redox flow batteries. Adv. Energy Mater. 5, 1–6 (2015).
Lall-Ramnarine, S. I. et al. Connecting structural and transport properties of ionic liquids with cationic oligoether chains. J. Electrochem. Soc. 164, H5247–H5262 (2017).
Gong, K., Fang, Q., Gu, S., Li, S. F. Y. & Yan, Y. Nonaqueous redox-flow batteries: organic solvents, supporting electrolytes, and redox pairs. Energy Environ. Sci. 8, 3515–3530 (2015).
Sevov, C. S., Hendriks, K. H. & Sanford, M. S. Low-potential pyridinium anolyte for aqueous redox flow batteries. J. Phys. Chem. C 121, 24376–24380 (2017).
Cheng, W. C. & Kurth, M. J. The Zincke reaction. A review. Org. Prep. Proced. Int. 34, 585–608 (2002).
DiMauro, E. F. & Kozlowski, M. C. Phosphabenzenes as electron withdrawing phosphine ligands in catalysis. J. Chem. Soc. Perkin. Trans. 2, 439–444 (2002).
Yue, H. et al. Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci. 10, 4430–4435 (2019).
Abraham, M. H. & Le, J. The correlation and prediction of the solubility of compounds in water using an amended solvation energy relationship. J. Pharm. Sci. 88, 868–880 (1999).
Brethomé, A. V., Fletcher, S. P. & Paton, R. S. Conformational effects on physical-organic descriptors: the case of sterimol steric parameters. ACS Catal. 9, 2313–2323 (2019).
Verloop, A., Hoogenstraaten, W. & Tipker, J. in Drug Design. (ed. Ariënsvol, E. J.) vol. 1962, 165–207 (Academic Press, 1976).
Verloop, A. in The Sterimol Approach: Further Development of the Method and New Applications (eds Doyle, P. & Fujita, T.) 339–344 (Elsevier, 1983).
Karthikeyan, S., Ramanathan, V. & Mishra, B. K. Influence of the substituents on the CH···π interaction: benzene–methane complex. J. Phys. Chem. A 117, 6687–6694 (2013).
Wheeler, S. E., Seguin, T. J., Guan, Y. & Doney, A. C. Noncovalent interactions in organocatalysis and the prospect of computational catalyst design. Acc. Chem. Res. 49, 1061–1069 (2016).
Neel, A. J., Hilton, M. J., Sigman, M. S. & Toste, F. D. Exploiting non-covalent π interactions for catalyst design. Nature 543, 637–646 (2017).
Houser, J. et al. The CH–π interaction in protein–carbohydrate binding: bioinformatics and in vitro quantification. Chem. Eur. J. 26, 10769–10780 (2020).
Tsuzuki, S., Honda, K., Uchimaru, T., Mikami, M. & Tanabe, K. The magnitude of the CH/π interaction between benzene and some model hydrocarbons. J. Am. Chem. Soc. 122, 3746–3753 (2000).
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: Links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic Press, 2011).
Mclachlan, A. D. Effect of the medium on dispersion forces in liquids. Discuss. Faraday Soc. 40, 239–245 (1965).
Davey, R. J., Schroeder, S. L. M. & Ter Horst, J. H. Nucleation of organic crystals—a molecular perspective. Angew. Chem. Int. Ed. 52, 2166–2179 (2013).
Hulme, A. T. et al. Search for a predicted hydrogen bonding motif—a multidisciplinary investigation into the polymorphism of 3-azabicyclo[3.3.1]nonane-2,4-dione. J. Am. Chem. Soc. 129, 3649–3657 (2007).
Davey, R. J., Dent, G., Mughal, R. K. & Parveen, S. Concerning the relationship between structural and growth synthons in crystal nucleation: solution and crystal chemistry of carboxylic acids as revealed through IR spectroscopy. Crystal Growth Design 6, 1788–1796 (2006).
Tresca, B. W. et al. Substituent effects in CH hydrogen bond interactions: linear free energy relationships and influence of anions. J. Am. Chem. Soc. 137, 14959–14967 (2015).
Simeral, L. & Amey, R. L. Dielectric properties of liquid propylene carbonate. J. Phys. Chem. 74, 1443–1446 (1970).
Maryott, A. A. & Smith, E. Table of Dielectric Constants of Pure Liquids. 514, 1–56 (US Government Printing Office, 1951).
Kolling, O. W. Dielectric characterization of cosolvent systems containing tetrahydrofuran. Trans. Kansas Acad. Sci. 94, 107 (1991).
Richards, T. W. & Shipley, J. W. The dielectric constants of typical aliphatic and aromatic hydrocarbons, cyclohexane, cyclohexanone, and cyclohexanol. J. Am. Chem. Soc. 41, 2002–2012 (1919).
Pinal, R. Effect of molecular symmetry on melting temperature and solubility. Org. Biomol. Chem. 2, 2692–2699 (2004).
Umezawa, Y., Tsuboyama, S., Honda, K., Uzawa, J. & Nishio, M. CH/π interaction in the crystal structure of organic compounds. A database study. Bull. Chem. Soc. Jpn 71, 1207–1213 (1998).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Kawahara, S. I., Tsuzuki, S. & Uchimaru, T. Theoretical study of the C-F/π interaction: attractive interaction between fluorinated alkane and an electron-deficient π-system. J. Phys. Chem. A 108, 6744–6749 (2004).
Wheeler, S. E. & Houk, K. N. Substituent effects in cation/π interactions and electrostatic potentials above the centers of substituted benzenes are due primarily to through-space effects of the substituents. J. Am. Chem. Soc. 131, 3126–3127 (2009).
Sinnokrot, M. O. & Sherrill, C. D. Substituent effects in π–π interactions: sandwich and t-shaped configurations. J. Am. Chem. Soc. 126, 7690–7697 (2004).
Acknowledgements
We thank the Michigan State University Center for Crystallographic Research for crystallographic analysis and the Michigan State University Mass Spectrometry and Metabolomics Core for providing high-resolution mass spectra of pyridinium salts. The Rigaku Synergy S Diffractometer was purchased with support from the MRI program by the National Science Foundation under grant no. 1919565 for use at the Center for Crystallographic Research, MSU. Portions of this project were made possible by a grant from the Community Foundation of Holland/Zeeland and Lakeshore Advantage through a Michigan Strategic Fund grant (T.G. and C.H.).
Author information
Authors and Affiliations
Contributions
S.S., T.G. and D.P.H. were responsible for the conceptualization of the project. C.H. performed all synthetic procedures. S.S. performed all solubility measurements and crystal growth. L.M. and D.P.H. performed all DFT calculations. R.J.S. collected and analysed all the crystallographic data. S.S., C.H., C.B. and N.G.G.C. analysed and interpreted the experimental and computational data. S.S., C.H., L.M. and D.P.H. prepared the Supplementary Information. S.S. and D.P.H. drafted the original version of the manuscript. All authors contributed to reviewing and editing the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Magdaléna Hromadova, Steve Scheiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 C-H···π interactions correlate with pyridinium solubility in several aprotic solvents.
Plots comparing solubilities of nine representative pyridinium derivatives from low (1, 2, 6, 16), moderate (5, 10, 12, 20) and high (14) solubility regimes (in acetonitrile). Solubilities were evaluated in acetonitrile (blue circles), THF (purple diamonds), cyclohexanone (green triangles), and propylene carbonate (orange squares). (A) A plot comparing pyridinium solubility in each solvent to a descriptor of C-H···π interactions (∑d−6). (B) Semi-log plot of pyridinium solubility in each solvent vs. the dielectric constant of the respective solvent (that is, εTHF = 7.6, εcyclohexanone = 18, εacetonitrile = 37, and εPC = 66). Solubility measurements were performed at 22 °C. Average values are reported where error bars represent one standard deviation with n = 3 for all compounds except for compound 17 (n = 2), compound 24 (n = 5), and compound 14 (n = 9).
Supplementary information
Supplementary Information
Supplementary materials and methods, text, Figs. 1–97 and Tables 1–6.
Supplementary Data 1
Crystallographic data for compound 1; CCDC reference 2157789.
Supplementary Data 2
Crystallographic data for compound 2; CCDC reference 2156178.
Supplementary Data 3
Crystallographic data for compound 4; CCDC reference 2156211.
Supplementary Data 4
Crystallographic data for compound 5; CCDC reference 2167771.
Supplementary Data 5
Crystallographic data for compound 6; CCDC reference 2155765.
Supplementary Data 6
Crystallographic data for compound 7; CCDC reference 2163473.
Supplementary Data 7
Crystallographic data for compound 8; CCDC reference 2157813.
Supplementary Data 8
Crystallographic data for compound 9; CCDC reference 2156206.
Supplementary Data 9
Crystallographic data for compound 10; CCDC reference 2157809.
Supplementary Data 10
Crystallographic data for compound 11; CCDC reference 2155982.
Supplementary Data 11
Crystallographic data for compound 12; CCDC reference 2155992.
Supplementary Data 12
Crystallographic data for compound 13; CCDC reference 2156563.
Supplementary Data 13
Crystallographic data for compound 14; CCDC reference 2155715.
Supplementary Data 14
Crystallographic data for compound 15; CCDC reference 2166708.
Supplementary Data 15
Crystallographic data for compound 16; CCDC reference 2162102.
Supplementary Data 16
Crystallographic data for compound 17; CCDC reference 2155704.
Supplementary Data 17
Crystallographic data for compound 18; CCDC reference 2156214.
Supplementary Data 18
Crystallographic data for compound 19; CCDC reference 2158012.
Supplementary Data 19
Crystallographic data for compound 20; CCDC reference 2168227.
Supplementary Data 20
Crystallographic data for compound 21; CCDC reference 2162134.
Supplementary Data 21
Crystallographic data for compound 22; CCDC reference 2163981.
Supplementary Data 22
Crystallographic data for compound 23; CCDC reference 2165226.
Supplementary Data 1
Crystallographic structure factor data for compound 1; CCDC reference 2157789.
Supplementary Data 2
Crystallographic structure factor data for compound 2; CCDC reference 2156178.
Supplementary Data 3
Crystallographic structure factor data for compound 4; CCDC reference 2156211.
Supplementary Data 4
Crystallographic structure factor data for compound 5; CCDC reference 2167771.
Supplementary Data 5
Crystallographic structure factor data for compound 6; CCDC reference 2155765.
Supplementary Data 6
Crystallographic structure factor data for compound 7; CCDC reference 2163473.
Supplementary Data 7
Crystallographic structure factor data for compound 8; CCDC reference 2157813.
Supplementary Data 8
Crystallographic structure factor data for compound 9; CCDC reference 2156206.
Supplementary Data 9
Crystallographic structure factor data for compound 10; CCDC reference 2157809.
Supplementary Data 10
Crystallographic structure factor data for compound 11; CCDC reference 2155982.
Supplementary Data 11
Crystallographic structure factor data for compound 12; CCDC reference 2155992.
Supplementary Data 12
Crystallographic structure factor data for compound 13; CCDC reference 2156563.
Supplementary Data 13
Crystallographic structure factor data for compound 14; CCDC reference 2155715.
Supplementary Data 14
Crystallographic structure factor data for compound 15; CCDC reference 2166708.
Supplementary Data 15
Crystallographic structure factor data for compound 16; CCDC reference 2162102.
Supplementary Data 16
Crystallographic structure factor data for compound 17; CCDC reference 2155704.
Supplementary Data 17
Crystallographic structure factor data for compound 18; CCDC reference 2156214.
Supplementary Data 18
Crystallographic structure factor data for compound 19; CCDC reference 2158012.
Supplementary Data 19
Crystallographic structure factor data for compound 20; CCDC reference 2168227.
Supplementary Data 20
Crystallographic structure factor data for compound 21; CCDC reference 2162134.
Supplementary Data 21
Crystallographic structure factor data for compound 22; CCDC reference 2163981.
Supplementary Data 22
Crystallographic structure factor data for compound 23; CCDC reference 2165226.
Source data
Source Data Fig. 2
Pyridinium solubilities in acetonitrile.
Source Data Fig. 3
Representative cyclic voltammograms. Experimentally determined reduction potentials. Experimentally versus computationally determined reduction potentials.
Source Data Fig. 4
Pyridinium solubilities in acetonitrile versus polarizability. Pyridinium solubilities in acetonitrile versus dipole moment. Pyridinium solubilities in acetonitrile versus sterimol parameters. Pyridinium solubilities in acetonitrile versus average distance between N+ and B−.
Source Data Fig. 5
Pyridinium solubilities in acetonitrile versus CH–π parameter.
Source Data Fig. 6
Variable concentration H-NMR peak change for compound 14. Variable concentration H-NMR peak change for compound 13.
Source Data Extended Data Fig.1/Table 1
Pyridinium solubilities in acetonitrile, cyclohexanone, PC and THF versus CH−π parameter. Pyridinium solubilities in acetonitrile, cyclohexanone, PC and THF versus solvent dielectric constant.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samaroo, S., Hengesbach, C., Bruggeman, C. et al. C–H···π interactions disrupt electrostatic interactions between non-aqueous electrolytes to increase solubility. Nat. Chem. 15, 1365–1373 (2023). https://doi.org/10.1038/s41557-023-01291-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01291-1
This article is cited by
-
A foggy day in London dispersion town
Nature Chemistry (2023)