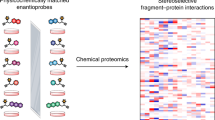
Abstract
Advances in chemoproteomic technology have revealed covalent interactions between small molecules and protein nucleophiles, primarily cysteine, on a proteome-wide scale. Most chemoproteomic screening approaches are indirect, relying on competition between electrophilic fragments and a minimalist electrophilic probe with inherently limited proteome coverage. Here we develop a chemoproteomic platform for direct electrophile-site identification based on enantiomeric pairs of clickable arylsulfonyl fluoride probes. Using stereoselective site modification as a proxy for ligandability in intact cells, we identify 634 tyrosines and lysines within functionally diverse protein sites, liganded by structurally diverse probes. Among multiple validated sites, we discover a chiral probe that modifies Y228 in the MYC binding site of the epigenetic regulator WDR5, as revealed by a high-resolution crystal structure. A distinct chiral probe stimulates tumour cell phagocytosis by covalently modifying Y387 in the recently discovered immuno-oncology target APMAP. Our work provides a deep resource of ligandable tyrosines and lysines for the development of covalent chemical probes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Uncropped gel images are provided as Source Data files accompanying this paper. The reported crystal structure has been deposited to the Protein Data Bank (PDB) under accession no. 8F93. All proteomic raw data have been deposited to MassIVE (http://massive.ucsd.edu) with accession no. MSV000090778, as well as in ProteomeXchange (http://www.proteomexchange.org) with accession no. PXD042307. Source data are provided with this paper.
Code availability
The script used for identifying modified sites and proximal ligands in the PDB has been deposited to GitHub (https://github.com/aacuesta/Ying2023NatChem).
References
Cross, D. A. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
Byrd, J. C. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013).
Hong, D. S. et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383, 1207–1217 (2020).
Cuesta, A., Wan, X., Burlingame, A. L. & Taunton, J. Ligand conformational bias drives enantioselective modification of a surface-exposed lysine on Hsp90. J. Am. Chem. Soc. 142, 3392–3400 (2020).
Wan, X. et al. Discovery of lysine-targeted eIF4E inhibitors through covalent docking. J. Am. Chem. Soc. 142, 4960–4964 (2020).
Dalton, S. E. et al. Selectively targeting the kinome-conserved lysine of PI3Kδ as a general approach to covalent kinase inhibition. J. Am. Chem. Soc. 140, 932–939 (2018).
Baggio, C. et al. Design of potent pan-IAP and Lys-covalent XIAP selective inhibitors using a thermodynamics driven approach. J. Med. Chem. 61, 6350–6363 (2018).
Bum-Erdene, K. et al. Small-molecule covalent bond formation at tyrosine creates a binding site and inhibits activation of Ral GTPases. Proc. Natl Acad. Sci. USA 117, 7131–7139 (2020).
Teng, M. et al. Rationally designed covalent BCL6 inhibitor that targets a tyrosine residue in the homodimer interface. ACS Med. Chem. Lett. 11, 1269–1273 (2020).
Hatcher, J. M. et al. SRPKIN-1: a covalent SRPK1/2 inhibitor that potently converts VEGF from pro-angiogenic to anti-angiogenic isoform. Cell Chem. Biol. 25, 460–470 (2018).
Mukherjee, H. et al. Discovery and optimization of covalent Bcl-xL antagonists. Bioorg. Med. Chem. Lett. 29, 126682 (2019).
Glukhova, A. et al. Structure of the adenosine A(1) receptor reveals the basis for subtype selectivity. Cell 168, 867–877 (2017).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Brulet, J. W., Borne, A. L., Yuan, K., Libby, A. H. & Hsu, K.-L. Liganding functional tyrosine sites on proteins using sulfur-triazole exchange chemistry. J. Am. Chem. Soc. 142, 8270–8280 (2020).
Abbasov, M. E. et al. A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem. 13, 1081–1092 (2021).
Hacker, S. M. et al. Global profiling of lysine reactivity and ligandability in the human proteome. Nat. Chem. 9, 1181–1190 (2017).
Kuljanin, M. et al. Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. Nat. Biotechnol. 39, 630–641 (2021).
McCloud, R. L. et al. Direct target site identification of a sulfonyl-triazole covalent kinase probe by LC-MS chemical proteomics. Anal. Chem. 93, 11946–11955 (2021).
Wang, Y. et al. Expedited mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs. Nat. Chem. 11, 1113–1123 (2019).
Vinogradova, E. V. et al. An activity-guided map of electrophile-cysteine interactions in primary human T cells. Cell 182, 1009–1026 (2020).
Tao, Y. et al. Targeted protein degradation by electrophilic PROTACs that stereoselectively and site-specifically engage DCAF1. J. Am. Chem. Soc. 144, 18688–18699 (2022).
Szychowski, J. et al. Cleavable biotin probes for labeling of biomolecules via azide-alkyne cycloaddition. J. Am. Chem. Soc. 132, 18351–18360 (2010).
Rabalski, A. J., Bogdan, A. R. & Baranczak, A. Evaluation of chemically-cleavable linkers for quantitative mapping of small molecule-cysteinome reactivity. ACS Chem. Biol. 14, 1940–1950 (2019).
Li, Z., Liu, K., Xu, P. & Yang, J. Benchmarking cleavable biotin tags for peptide-centric chemoproteomics. J Proteome Res. 21, 1349–1358 (2022).
Erickson, B. K. et al. Active instrument engagement combined with a real-time database search for improved performance of sample multiplexing workflows. J. Proteome Res. 18, 1299–1306 (2019).
Schweppe, D. K. et al. Full-featured, real-time database searching platform enables fast and accurate multiplexed quantitative proteomics. J. Proteome Res. 19, 2026–2034 (2020).
Cruite, J. T. et al. Cereblon covalent modulation through structure-based design of histidine targeting chemical probes. RSC Chem. Biol. 3, 1105–1110 (2022).
Tam, J. et al. An activity-based probe for high-throughput measurements of triacylglycerol lipases. Anal. Biochem. 414, 254–260 (2011).
Gambini, L. et al. Covalent inhibitors of protein-protein interactions targeting lysine, tyrosine or histidine residues. J. Med. Chem. 62, 5616–5627 (2019).
Narayanan, A. & Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 6, 2650–2659 (2015).
Zanon, P. R. A. et al. Profiling the proteome-wide selectivity of diverse electrophiles. Preprint at https://chemrxiv.org/engage/chemrxiv/article-details/60c755f2bb8c1a7d393dc505 (2021).
Gu, C. et al. Chemical proteomics with sulfonyl fluoride probes reveals selective labeling of functional tyrosines in glutathione transferases. Chem. Biol. 20, 541–548 (2013).
Mukherjee, H. et al. A study of the reactivity of S((VI))-F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 15, 9685–9695 (2017).
Brighty, G. J. et al. Using sulfuramidimidoyl fluorides that undergo sulfur(VI) fluoride exchange for inverse drug discovery. Nat. Chem. 12, 906–913 (2020).
Stanevich, V. et al. The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell 41, 331–342 (2011).
Hwang, J., Lee, J. A. & Pallas, D. C. Leucine carboxyl methyltransferase 1 (LCMT-1) methylates protein phosphatase 4 (PP4) and protein phosphatase 6 (PP6) and differentially regulates the stable formation of different PP4 holoenzymes. J. Biol. Chem. 291, 21008–21019 (2016).
De Baere, I. et al. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry 38, 16539–16547 (1999).
Reckel, S. et al. Structural and functional dissection of the DH and PH domains of oncogenic Bcr-Abl tyrosine kinase. Nat. Commun. 8, 2101 (2017).
Lee, J. H. & Skalnik, D. G. Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A Histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol. Cell. Biol. 28, 609–618 (2008).
Wu, M. et al. Molecular regulation of H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS. Mol. Cell. Biol. 28, 7337–7344 (2008).
Thomas, L. R. et al. Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol. Cell 58, 440–452 (2015).
Chacón Simon, S. et al. Discovery of WD repeat-containing protein 5 (WDR5)–MYC inhibitors using fragment-based methods and structure-based design. J. Med. Chem. 63, 4315–4333 (2020).
Tian, J. et al. Discovery and structure-based optimization of potent and selective WD repeat domain 5 (WDR5) inhibitors containing a dihydroisoquinolinone bicyclic core. J. Med. Chem. 63, 656–675 (2020).
Ding, J. et al. Discovery of potent small-molecule inhibitors of WDR5-MYC interaction. ACS Chem. Biol. 18, 34–40 (2023).
Kamber, R. A. et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature 597, 549–554 (2021).
Hahm, H. S. et al. Global targeting of functional tyrosines using sulfur-triazole exchange chemistry. Nat. Chem. Biol. 16, 150–159 (2020).
Chen, W. et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site Tyr residue. J. Am. Chem. Soc. 138, 7353–7364 (2016).
Mortenson, D. E. et al. Inverse drug discovery’ strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018).
Wang, N. et al. Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 (2018).
Hett, E. C. et al. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 10, 1094–1098 (2015).
Cuesta, A. & Taunton, J. Lysine-targeted inhibitors and chemoproteomic probes. Annu. Rev. Biochem. 88, 365–381 (2019).
Yang, T. et al. Reversible lysine-targeted probes reveal residence time-based kinase selectivity. Nat. Chem. Biol. 18, 934–941 (2022).
Metcalf, B. et al. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med. Chem. Lett. 8, 321–326 (2017).
Acknowledgements
Funding for this study was provided by the Ono Pharma Foundation (J.T.), Pfizer and the Tobacco-Related Disease Research Program Postdoctoral Fellowship Award (T32FT4880 to G.B.C.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank B. Küster for guidance on MS data acquisition and analysis.
Author information
Authors and Affiliations
Contributions
Y.C. and J.T. conceived the project, designed the experiments and analysed the data. S.Z. and Y.S.M. synthesized the SFs. Y.C. performed the chemoproteomic and validation experiments. G.B.C. solved the WDR5 X-ray structure and performed biochemical experiments with recombinant APMAP. A.C. wrote the script for identifying modified sites in the PDB. R.A.K. performed the phagocytosis assays, with input from M.C.B. Y.C. and J.T. wrote the manuscript, with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
J.T. is a cofounder of Kezar Life Sciences and Terremoto Biosciences, and a scientific advisor to Entos. M.C.B. and R.A.K. have outside interests in DEM Biopharma. S.Z. and Y.S.M. are employees of Enamine Ltd and Chemspace LLC, respectively. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Benjamin Cravatt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Volcano plots showing modified sites (MS2 and RTS-MS3 data) for probes 1–10.
a-j, Log2ratio((R)/(S)) is on the x-axis and –log10(P value) is on the y-axis. Modified sites are indicated as (R)-selective (blue), (S)-selective (orange), and nonselective (grey). P values were determined by Student’s t-test (two-tailed, two-sample equal variance).
Extended Data Fig. 2 Analysis of tyrosines and lysines modified by chiral sulfonyl fluoride probes.
a-f, Number of liganded and total Tyr and Lys identified in this study compared with the indicated previous studies14,15. g, The number of enantiomeric probe pairs (based on fragments 1-10) per site (x- axis) was plotted against the total number of sites modified by the indicated number of enantiomeric probe pairs (y-axis).
Extended Data Fig. 3 Functionally diverse protein sites targeted by chiral sulfonyl fluoride probes.
a-f, Structures of FUS, YBX1, KIF11, KDM1A, PRMT5, and SIRT2, highlighting the probe-modified Tyr proximal to bound ligands.
Extended Data Fig. 4 Probe modification of LCMT1.
a, HEK293T cells were transfected with Flag-LCMT1 and treated with SF probes (10 μM, 1 h). After cell lysis, anti-Flag enrichment, 3xFlag peptide elution and click reaction with TAMRA-azide, samples were analyzed by in-gel fluorescence and western blotting. NC indicates nontransfected cells. Data are representative of two independent experiments. b, HEK293T cells were transfected with WT or Y315F Flag- LCMT1 and treated with 4-SF probes (10 μM, 1 h). The cells were lysed and processed as described above. Data are representative of two independent experiments.
Extended Data Fig. 5 Probe modification of ABR.
a, HEK293T cells were transfected with Flag-ABR and treated with SF probes (10 μM, 1 h). After cell lysis, anti-Flag enrichment, 3xFlag peptide elution and click reaction with TAMRA-azide, samples were analyzed by in-gel fluorescence and western blotting. NC indicates nontransfected cells. Data are representative of two independent experiments. b, HEK293T cells were transfected with WT or Y344F Flag-ABR and treated with 7-SF probes (10 μM, 1 h). The cells were lysed and processed as described above. Data are representative of two independent experiments.
Extended Data Fig. 6 Probe modification of WDR82.
a, HEK293T cells were transfected with WT or Y102F Flag-WDR82 and treated with 7-SF probes (50 μM, 1 h). After cell lysis, anti- Flag enrichment, 3xFlag peptide elution and click reaction with TAMRA-azide, samples were analyzed by in-gel fluorescence and western blotting. Data are representative of two independent experiments. NC indicates nontransfected cells. b, Recombinant WT and Y102F WDR82 (0.44 μM) were incubated with 7-SF probes (40 μM, 37 °C, overnight) and analyzed by intact-protein mass spectrometry. Data are representative of two independent experiments. c, HEK293T cells were transiently transfected with Flag-WDR82 and treated with the indicated SF probes (50 μM, 1 h). The cells were lysed and processed as described above. Data are representative of two independent experiments. d, Overlay of the AlphaFold structure of WDR82 with the crystal structure of WDR5 bound to KANSL1 peptide (PDB: 4CY2).
Extended Data Fig. 7 Electron density map of (R)-2-SF.
Electron density (2Fo-Fc) map of (R)-2-SF is shown at a contour level of 1σ.
Extended Data Fig. 8 Data collection and refinement statistics.
Data collection and refinement statistics (molecular replacement) for WDR5-(R)-2-SF complex.
Extended Data Fig. 9 Chemoproteomic analysis of 1-SF probes in dose-response mode.
a, HEK293T cells that stably overexpress Flag-APMAP were treated separately with 0, 3.75, 7.5, 15, 30 and 60 µM of (R)-1-SF and (S)-1-SF for 1.5 h at 37 °C. Cell lysates were conjugated with biotin–DADPS-azide via click chemistry. After enrichment and trypsin digestion, probe-modified peptides from each sample were separately labeled with TMT 11-plex reagents. Probe-modified peptides from each sample were eluted with 2% formic acid, combined, and analyzed by LC-MS/MS. b-d, TMT reporter ion intensities were plotted as a function of probe concentration for peptides quantified by MS2 and RTS: APMAP Y387 (b), APMAP K347 (c), and MTHFD1 Y52 (d). e, Crystal structure (PDB: 1DIA) depicting MTHFD1 Y52 proximal to the inhibitor LY249543.
Extended Data Fig. 10 Antibody-dependent phagocytosis assay.
a, APMAPKO Ramos- Cas9 cells (rescued with Flag-APMAP WT, E103A and Y387F) were resuspended in SDS loading buffer containing 100 mM DTT, sonicated and heated (70 °C, 10 min). Samples were analyzed by western blotting. Data are representative of two independent experiments. b, Scheme of phagocytosis assay.
Supplementary information
Supplementary Information
Supplementary dataset legends, tables, biological methods, synthetic methods, NMR spectra, references.
Supplementary Data 1
MS data from TMT6 experiments. (Table 1) Modified sites and enantiomer modification ratios for 10 chiral sulfonyl fluoride probe pairs. (Tables 2–21) Each table displays the MS data, including TMT6 intensity values (Maxquant output), for sites modified by the indicated chiral sulfonyl fluoride probe pair (MS2 and RTS methods). P-values were determined by Student’s t-test (two-tailed, two-sample equal variance).
Supplementary Data 2
MS data from the 1-SF dose-response experiment (Maxquant output), related to Extended Data Fig. 9. (Table 1) MS2 method. (Table 2) RTS method.
Supplementary Data 3
Sequences of oligonucleotides used in this work.
Source data
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 10
Unprocessed western blots and/or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Craven, G.B., Kamber, R.A. et al. Direct mapping of ligandable tyrosines and lysines in cells with chiral sulfonyl fluoride probes. Nat. Chem. 15, 1616–1625 (2023). https://doi.org/10.1038/s41557-023-01281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01281-3
This article is cited by
-
Functionalizing tandem mass tags for streamlining click-based quantitative chemoproteomics
Communications Chemistry (2024)