Abstract
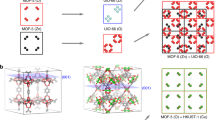
Interpenetrated metal–organic frameworks (MOFs) comprise two or more lattices that are mutually entangled. Interpenetration can be used to tune the structures and pore architectures of MOFs to influence, for example, their stability or interactions with guest molecules. The interpenetrating sublattices are typically identical, but hetero-interpenetrated MOFs, which consist of sublattices that are different from one another, have also been serendipitously produced. Here we describe a strategy for the deliberate synthesis of hetero-interpenetrated MOFs. We use the cubic α-MUF-9 framework as a host sublattice to template the growth of a second sublattice within its pores. Three different secondary sublattices are grown—two of which are not known as standalone MOFs—leading to three different hetero-interpenetrated MOFs. This strategy may serve to combine different properties into one material. We produce an asymmetric catalysis by allocating separate roles to the interpenetrating sublattices in a hetero-interpenetrated MOF: an achiral secondary amine on one sublattice provides the catalytic activity, while the chiral α-MUF-10 host imparts asymmetry to aldol and Henry reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2144458–2144473 and 2269651 (MUF-91), 2144574–2144588 (MUF-92), 2149445–2149471 and 2269651 (MUF-93) and 2149424–2149437 (rastering files for MUF-93), as detailed in Supplementary Table 1. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are included in the Supplementary Information. Source data are provided with this article and also available on figshare, https://doi.org/10.6084/m9.figshare.22682965.v2 (ref. 28).
Code availability
The Python scripts are available on figshare at https://doi.org/10.6084/m9.figshare.22682965.v2 (ref. 28).
References
Batten, S. R. Topology of interpenetration. CrystEngComm 3, 67–72 (2001).
Batten, S. R. & Robson, R. Interpenetrating nets: ordered, periodic entanglement. Angew. Chem. Int. Ed. 37, 1460–1494 (1998).
Haldar, R., Sikdar, N. & Maji, T. K. Interpenetration in coordination polymers: structural diversities toward porous functional materials. Mater. Today 18, 97–116 (2015).
Jiang, H.-L., Makal, T. A. & Zhou, H.-C. Interpenetration control in metal-organic frameworks for functional applications. Coord. Chem. Rev. 257, 2232–2249 (2013).
Park, J. H. et al. Interpenetration control, sorption behavior, and framework flexibility in Zn(II) metal-organic frameworks. Cryst. Growth Des. 14, 699–704 (2014).
Verma, G., Butikofer, S., Kumar, S. & Ma, S. Regulation of the degree of interpenetration in metal–organic frameworks. Top. Curr. Chem. 378, 4 (2019).
Deshpande, R. K., Minnaar, J. L. & Telfer, S. G. Thermolabile groups in metal–organic frameworks: suppression of network interpenetration, post-synthetic cavity expansion and protection of reactive functional groups. Angew. Chem. Int. Ed. 47, 4598–4602 (2010).
Miller, J. S. Interpenetrating lattices—materials of the future. Adv. Mater. 13, 525–527 (2001).
Li, S. et al. A 2D metal-organic framework interpenetrated by a 2D supramolecular framework assembled by CH/ π interactions. Inorg. Chem. Commun. 130, 108705 (2021).
Zhang, M.-D. et al. Chiral 3D/3D hetero-interpenetrating framework with six kinds of helices, 3D polyrotaxane and 2D network via one-pot reaction. CrystEngComm 15, 227–230 (2013).
Xu, H. et al. An unprecedented 3D/3D hetero-interpenetrated MOF built from two different nodes, chemical composition, and topology of networks. CrystEngComm 14, 5720–5722 (2012).
Batten, S. R. in Metal-Organic Frameworks: Design and Application (ed MacGillivray, L. R.) Ch. 3 (John Wiley & Sons, 2010).
Kwon, O., Park, S., Zhou, H. C. & Kim, J. Computational prediction of hetero-interpenetration in metal-organic frameworks. Chem. Commun. 53, 1953–1956 (2017).
Sezginel, K., Feng, T. & Wilmer, C. Discovery of hypothetical hetero-interpenetrated MOFs with arbitrarily dissimilar topologies and unit cell shapes. CrystEngComm 19, 4497–4504 (2017).
Furukawa, H., Muller, U. & Yaghi, O. M. “Heterogeneity within order” in metal-organic frameworks. Angew. Chem. Int. Ed. 54, 3417–3430 (2015).
Pang, Q., Tu, B. & Li, Q. Metal-organic frameworks with multicomponents in order. Coord. Chem. Rev. 388, 107–125 (2019).
Ferguson, A. et al. Controlled partial interpenetration in metal–organic frameworks. Nat. Chem 8, 250–257 (2016).
Verma, G. et al. Partially interpenetrated NbO topology metal–organic framework exhibiting selective gas adsorption. Cryst. Growth Des. 17, 2711–2717 (2017).
Yang, S. et al. A partially interpenetrated metal–organic framework for selective hysteretic sorption of carbon dioxide. Nat. Mater. 11, 710–716 (2012).
O’Nolan, D. et al. Impact of partial interpenetration in a hybrid ultramicroporous material on C2H2/C2H4 separation performance. Chem. Commun. 54, 3488–3491 (2018).
Pan, L., Ching, N., Huang, X. & Li, J. Reactions and reactivity of Co−bpdc coordination polymers (bpdc = 4,4′-biphenyldicarboxylate). Inorg. Chem. 39, 5333–5340 (2000).
Hausdorf, S., Baitalow, F., Böhle, T., Rafaja, D. & Mertens, F. O. Main-group and transition-element IRMOF homologues. J. Am. Chem. Soc. 132, 10978–10981 (2010).
Brozek, C. K., Cozzolino, A. F., Teat, S. J., Chen, Y.-S. & Dincă, M. Quantification of site-specific cation exchange in metal–organic frameworks using multi-wavelength anomalous X-ray dispersion. Chem. Mater. 25, 2998–3002 (2013).
Freedman, D. E. et al. Site specific X-ray anomalous dispersion of the geometrically frustrated kagomé‚ magnet, herbertsmithite, ZnCu3(OH)6Cl2. J. Am. Chem. Soc. 132, 16185–16190 (2010).
Helliwell, M. et al. Determination of zinc incorporation in the Zn-substituted gallophosphate ZnULM-5 by multiple wavelength anomalous dispersion techniques. Acta Cryst.B 66, 345–357 (2010).
Zhou, T.-Y., Auer, B., Lee, S. J. & Telfer, S. G. Catalysts confined in programmed framework pores enable new transformations and tune reaction efficiency and selectivity. J. Am. Chem. Soc. 141, 1577–1582 (2019).
Ishikawa, H. & Shiomi, S. Alkaloid synthesis using chiral secondary amine organocatalysts. Org. Biomol. Chem. 14, 409–424 (2016).
Perl, D. et al. Data for paper ‘Hetero-interpenetrated metal-organic frameworks’. figshare https://doi.org/10.6084/m9.figshare.22682965.v2 (2023).
Acknowledgements
This research was undertaken in part using the MX2 beamline at the Australian Synchrotron, part of the Australian Nuclear Science and Technology Organisation. We made use of the Australian Cancer Research Foundation detector, partially funded by the New Zealand Group and supported by Massey University. We are grateful to beamline scientists S. Panjikar and J. Price for their expert help. We also thank D. Lun for technical assistance and P. Plieger for guidance on AA spectroscopy. We received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
S.G.T. conceived the idea. D.P. developed the concept, conducted experiments, analysed the results and prepared the Supplementary Information. A.F. and S.J.L. conducted experimental work. G.B.J. conceived and advised on the anomalous scattering experiments. S.G.T. and G.B.J. supervised the project. S.G.T. and D.P. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Hai-Long Jiang, Hong-Cai Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30, Tables 1–8 and Methods.
Supplementary Data 1
Crystallographic information files (CIFs) for MUF-91 materials, [Zn4O(L1)3] and [Zn4O(bpdc)3]x.
Supplementary Data 2
CIFs for MUF-92 materials, [Zn4O(L1)3] and [Zn4O(bpdc-NH2)3]x.
Supplementary Data 3
CIFs for MUF-93 materials, [Zn4O(L1)3] and [Co4O(bpdc)3]x.
Supplementary Data 4
Rastering CIFs for MUF-93 materials, [Zn4O(L1)3] and [Co4O(bpdc)3]x.
Supplementary Data 5
Crystallographic files for difference datasets using two different wavelengths.
Supplementary Data 6
Source data for Supplementary Figs. 2, 6, 7, 12, 13a, 14, 16, 26, 27, 28 and 30.
Source data
Source Data Fig. 1
Source data for Figs. 1c–e.
Source Data Fig. 3
Source data for Fig. 3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perl, D., Lee, S.J., Ferguson, A. et al. Hetero-interpenetrated metal–organic frameworks. Nat. Chem. 15, 1358–1364 (2023). https://doi.org/10.1038/s41557-023-01277-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01277-z
This article is cited by
-
Interweaving different metal–organic frameworks
Nature Chemistry (2023)