Abstract
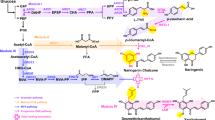
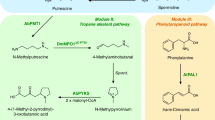
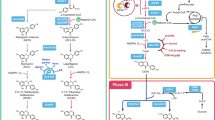
Obesity is a major health risk still lacking effective pharmacological treatment. A potent anti-obesity agent, celastrol, has been identified in the roots of Tripterygium wilfordii. However, an efficient synthetic method is required to better explore its biological utility. Here we elucidate the 11 missing steps for the celastrol biosynthetic route to enable its de novo biosynthesis in yeast. First, we reveal the cytochrome P450 enzymes that catalyse the four oxidation steps that produce the key intermediate celastrogenic acid. Subsequently, we show that non-enzymatic decarboxylation-triggered activation of celastrogenic acid leads to a cascade of tandem catechol oxidation-driven double-bond extension events that generate the characteristic quinone methide moiety of celastrol. Using this acquired knowledge, we have developed a method for producing celastrol starting from table sugar. This work highlights the effectiveness of combining plant biochemistry with metabolic engineering and chemistry for the scalable synthesis of complex specialized metabolites.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this article is available as a Supplementary Information file. The T. wilfordii cDNA sequences identified here have been deposited in GenBank under accession numbers OP970829 to OP970833. Source data are provided with this paper.
References
Olshansky, S. J. et al. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 352, 1138–1145 (2005).
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R. & Ozcan, U. Treatment of obesity with celastrol. Cell 161, 999–1011 (2015).
Ma, X. et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 22, 695–708 (2015).
Hu, M. et al. Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol. Cell 66, 141–153.e6 (2017).
Peng, S. Z. et al. Phase separation of Nur77 mediates celastrol-induced mitophagy by promoting the liquidity of p62/SQSTM1 condensates. Nat. Commun. 12, 5989 (2021).
Feng, X. et al. IL1R1 is required for celastrol’s leptin-sensitization and antiobesity effects. Nat. Med. 25, 575–582 (2019).
Grant, P. K., Johnson, A.W., Juby, P. F. & King, T. J. 114. Pristimerin. Part III. A modified structure for the chromophore. J. Chem. Soc. 0, 549–555 (1960).
Chou, T. Q. & Mei, P. F. The principle of the Chinese drug Lei-Kung-Teng, Tripterygium wilfordii, Hook. I. The coloring substance and the sugars. Chin. J. Physiol. 10, 529–534 (1936).
Kupchan, S. M., Court, W. A., Dailey, R. G. Jr, Gilmore, C. J. & Bryan, R. F. Tumor inhibitors. LXXIV. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 94, 7194–7195 (1972).
Hansen, N. L. et al. Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide. Nat. Commun. 13, 5011 (2022).
Tu, L. et al. Genome of Tripterygium wilfordii and identification of cytochrome P450 involved in triptolide biosynthesis. Nat. Commun. 11, 971 (2020).
Camelio, A. M., Johnson, T. C. & Siegel, D. Total synthesis of celastrol, development of a platform to access celastroid natural products. J. Am. Chem. Soc. 137, 11864–11867 (2015).
Li, S., Li, Y. & Smolke, C. D. Strategies for microbial synthesis of high-value phytochemicals. Nat. Chem. 10, 395–404 (2018).
Luo, X. et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126 (2019).
Srinivasan, P. & Smolke, C. D. Biosynthesis of medicinal tropane alkaloids in yeast. Nature 585, 614–619 (2020).
Paddon, C. J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532 (2013).
Galanie, S., Thodey, K., Trenchard, I. J., Interrante, M. F. & Smolke, C. D. Complete biosynthesis of opioids in yeast. Science 349, 1095–1100 (2015).
Zhou, J. et al. Friedelane-type triterpene cyclase in celastrol biosynthesis from Tripterygium wilfordii and its application for triterpenes biosynthesis in yeast. New Phytol. 223, 722–735 (2019).
Hansen, N. L. et al. Integrating pathway elucidation with yeast engineering to produce polpunonic acid the precursor of the anti-obesity agent celastrol. Microb. Cell Fact. 19, 15 (2020).
Pateraki, I., Heskes, A. M. & Hamberger, B. Cytochromes P450 for Terpene Functionalisation and Metabolic Engineering, in Biotechnology of Isoprenoids (eds Schrader, J. & Bohlmann, J.) (Springer, 2015).
Bach, S. S. et al. High-throughput testing of terpenoid biosynthesis candidate genes using transient expression in Nicotiana benthamiana. Methods Mol. Biol. 1153, 245–255 (2014).
Bruckner, K. & Tissier, A. High-level diterpene production by transient expression in Nicotiana benthamiana. Plant Methods 9, 46 (2013).
Li, K., Duan, H., Kawazoe, K. & Takaishi, Y. Terpenoids from Tripterygium wilfordii. Phytochemistry 45, 791–796 (1997).
Zhang, W., Pan, D., Zhang, L. & Shao, Y. Triterpenoids of Tripterygium wilfordii Hook-F. Acta Pharm. Sin. 21, 592–598 (1986).
Chen, K. et al. Anti-AIDS agents, 6. Salaspermic acid, an anti-HIV principle from Tripterygium wilfordii, and the structure–activity correlation with its related compounds. J. Nat. Prod. 55, 340–346 (1992).
Grant, J. L., Hsieh, C. H. & Makris, T. M. Decarboxylation of fatty acids to terminal alkenes by cytochrome P450 compound I. J. Am. Chem. Soc. 137, 4940–4943 (2015).
Grant, J. L., Mitchell, M. E. & Makris, T. M. Catalytic strategy for carbon–carbon bond scission by the cytochrome P450 OleT. Proc. Natl Acad. Sci. USA 113, 10049–10054 (2016).
Meunier, B., de Visser, S. P. & Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem. Rev. 104, 3947–3980 (2004).
Ren, D., Kim, M., Wang, S. A. & Liu, H. W. Identification of a pyrrole intermediate which undergoes C-glycosidation and autoxidation to yield the final product in showdomycin biosynthesis. Angew. Chem. Int. Ed. 60, 17148–17154 (2021).
Hong, B. et al. Biosynthesis of strychnine. Nature 607, 617–622 (2022).
Tahir, M. N., Shahbazi, F., Rondeau-Gagné, S. & Trant, J. F. The biosynthesis of the cannabinoids. J. Cannabis Res. 3, 7 (2021).
Zi, J. & Peters, R. J. Characterization of CYP76AH4 clarifies phenolic diterpenoid biosynthesis in the Lamiaceae. Org. Biomol. Chem. 11, 7650–7652 (2013).
Bolton, J. L. & Shen, L. p-Quinone methides are the major decomposition products of catechol estrogen o-quinones. Carcinogenesis 17, 925–929 (1996).
Wang, T. et al. Comparative analysis of four terpenoids in root and cortex of Tripterygium wilfordii Radix by different drying methods. BMC Complement. Altern. Med. 16, 476 (2016).
Arnold, R. T., Elmer, O. C. & Dodson, R. Thermal decarboxylation of unsaturated acids. J. Am. Chem. Soc. 72, 4359–4361 (1950).
Li, X., Li, Z. & Sun, J. Quinone methides and indole imine methides as intermediates in enantioselective catalysis. Nat. Synth. 1, 426–438 (2022).
Yang, J., Stuart, M. A. C. & Kamperman, M. Jack of all trades: versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 43, 8271–8298 (2014).
Valgimigli, L. et al. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 73, 1830–1841 (2008).
Luo, D. Q., Wang, H., Tian, X., Shao, H. J. & Liu, J. K. Antifungal properties of pristimerin and celastrol isolated from Celastrus hypoleucus. Pest Manag. Sci. 61, 85–90 (2005).
Abdel-Halim, M. S., Askoura, M., Mansour, B., Yahya, G. & El-Ganiny, A. M. In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates. J. Antibiot. 75, 679–690 (2022).
Yu, J. S. et al. Celastrol inhibits dengue virus replication via up-regulating type I interferon and downstream interferon-stimulated responses. Antivir. Res. 137, 49–57 (2017).
Padilla-Montaño, N., de León Guerra, L. & Moujir, L. Antimicrobial activity and mode of action of celastrol, a nortriterpen quinone isolated from natural sources. Foods 10, 591 (2021).
Lange, B. M. et al. Integrative approaches for the identification and localization of specialized metabolites in Tripterygium roots. Plant Physiol. 173, 456–469 (2016).
Vemishetti, P., Gibson, F. S. & Dillon, J. L. Semi-synthesis of paclitaxel using dialkyldichlorosilanes. US patent 6242614B1 (2001).
Li, B. J. et al. Improving 10-deacetylbaccatin III-10-β-O-acetyltransferase catalytic fitness for Taxol production. Nat. Commun. 8, 15544 (2017).
Vukovic, J. & Goodbody, A. E. Production of alkaloid dimers using ferric ion. US patent 4778885A (1986).
Zhang, J. et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature 609, 341–347 (2022).
Zhu, Z. T. et al. Metabolic compartmentalization in yeast mitochondria: burden and solution for squalene overproduction. Metab. Eng. 68, 232–245 (2021).
Zhu, Y. et al. High-yield production of protopanaxadiol from sugarcane molasses by metabolically engineered Saccharomyces cerevisiae. Microb. Cell Fact. 21, 230 (2022).
Wang, P. et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high efficiency. Cell Discov. 5, 5 (2019).
Wang, P., Wang, J., Zhao, G., Yan, X. & Zhou, Z. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound K. Synth. Syst. Biotechnol. 6, 69–76 (2021).
Liu, G. S. et al. The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction. Metab. Eng. 57, 151–161 (2020).
Dusséaux, S., Wajn, W. T., Liu, Y., Ignea, C. & Kampranis, S. C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl Acad. Sci. USA 117, 31789–31799 (2020).
Hansen, N. L. et al. The terpene synthase gene family in Tripterygium wilfordii harbors a labdane-type diterpene synthase among the monoterpene synthase TPS-b subfamily. Plant J. 89, 429–441 (2017).
Forman, V. et al. A gene cluster in Ginkgo biloba encodes unique multifunctional cytochrome P450s that initiate ginkgolide biosynthesis. Nat. Commun. 13, 5143 (2022).
De La Peña, R. & Sattely, E. S. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat. Chem. Biol. 17, 205–212 (2021).
Bach, S. S. et al. in Plant Isoprenoids: Methods and Protocols (ed. Rodríguez-Concepción, M.) 245–255 (Springer, 2014).
Jensen, K. & Møller, B. L. Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 71, 132–141 (2010).
Gietz, R. D. & Schiestl, R. H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007).
Acknowledgements
We thank F. Geu-Flores (University of Copenhagen), V. Roussis and E. Ioannou (National and Kapodistrian University of Athens, Greece) for critical reading of the manuscript, and H. Chen (Kunming University of Science and Technology, China) and H. Zhang (Swiss Federal Institute of Technology Lausanne, Switzerland) for discussions on the chemical mechanism. We also thank D. R. Nelson (University of Tennessee, USA) for assigning the CYP names. We thank J. Olsen, M. Ramirez, D. Pattison, I. Ovejero-Lopez and L. Kjærulff (University of Copenhagen) for their assistance in running analytical instruments. This work was financially supported by the Novo Nordisk Foundation (grants NNF17OC0027646 to S.B. and S.C.K. and NNF16OC0021760 and NNF19OC0055204 to S.C.K.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.Z., K.M., B.L.M., S.B. and S.C.K. conceived and designed the experiments. Y.Z., N.L.H. and M.P. conducted all cloning, the engineering of N. benthamiana and S. cerevisiae, and analysed the chromatography data. Y.Z. and Y.-T.D. isolated the products from S. cerevisiae and T. wilfordii roots. Y.Z. and D.S. analysed the NMR data. M.S.M. proposed the chemical mechanism. Y.Z., I.P., K.M. and S.C.K. wrote the paper. All authors contributed to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
S.C.K., Y.Z., K.M. and N.L.H. are co-inventors in a patent application (European Patent Office no. P101284EP00, 2022) describing the bioproduction of celastrol in yeast. The other authors claim no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Jens Nielsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–35 and Tables 1–7.
Supplementary Data 1
Oligo sequences used in this study.
Supplementary Data 2
Source data for Supplementary Figs. 6 and 7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Hansen, N.L., Duan, YT. et al. Biosynthesis and biotechnological production of the anti-obesity agent celastrol. Nat. Chem. 15, 1236–1246 (2023). https://doi.org/10.1038/s41557-023-01245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01245-7
This article is cited by
-
Engineering yeast to produce plant-derived anti-obesity agent
Nature Chemistry (2023)