Abstract
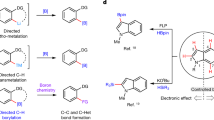
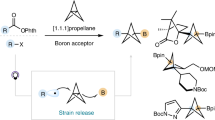
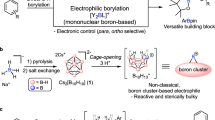
Catalytic borylations of sp3 C–H bonds occur with high selectivities for primary C–H bonds or secondary C–H bonds that are activated by nearby electron-withdrawing substituents. Catalytic borylation at tertiary C–H bonds has not been observed. Here we describe a broadly applicable method for the synthesis of boron-substituted bicyclo[1.1.1]pentanes and (hetero)bicyclo[2.1.1]hexanes by an iridium-catalysed borylation of the bridgehead tertiary C–H bond. This reaction is highly selective for the formation of bridgehead boronic esters and is compatible with a broad range of functional groups (>35 examples). The method is applicable to the late-stage modification of pharmaceuticals containing this substructure and the synthesis of novel bicyclic building blocks. Kinetic and computational studies suggest that C–H bond cleavage occurs with a modest barrier and that the turnover-limiting step of this reaction is an isomerization that occurs prior to reductive elimination that forms the C–B bond.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Complete experimental procedures, computational details and compound characterization data are available in the Supplementary Information. Atomic coordinates of optimized structures are available as Supplementary Data 1.
References
Liskey, C. W. & Hartwig, J. F. Iridium-catalyzed borylation of secondary C–H Bonds in cyclic ethers. J. Am. Chem. Soc. 134, 12422–12425 (2012).
Liskey, C. W. & Hartwig, J. F. Iridium-catalyzed C–H borylation of cyclopropanes. J. Am. Chem. Soc. 135, 3375–3378 (2013).
Li, Q., Liskey, C. W. & Hartwig, J. F. Regioselective borylation of the C–H bonds in alkylamines and alkyl ethers. Observation and origin of high reactivity of primary C–H bonds beta to nitrogen and oxygen. J. Am. Chem. Soc. 136, 8755–8765 (2014).
Jones, M. R., Fast, C. D. & Schley, N. D. Iridium-catalyzed sp3 C–H borylation in hydrocarbon solvent enabled by 2,2′-dipyridylarylmethane ligands. J. Am. Chem. Soc. 142, 6488–6492 (2020).
Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 368, 736 (2020).
Kawazu, R., Torigoe, T. & Kuninobu, Y. Iridium-catalyzed C(sp3)−H borylation using silyl-bipyridine pincer ligands. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202202327
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 8, 43–48 (2017).
Auberson, Y. P. et al. Improving nonspecific binding and solubility: bicycloalkyl groups and cubanes as para-phenyl bioisosteres. ChemMedChem 12, 590–598 (2017).
Pu, Q. et al. Discovery of potent and orally available bicyclo[1.1.1]pentane-derived indoleamine-2,3-dioxygenase 1 (IDO1) inhibitors. ACS Med. Chem. Lett. 11, 1548–1554 (2020).
Makarov, I. S., Brocklehurst, C. E., Karaghiosoff, K., Koch, G. & Knochel, P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem. Int. Ed. 56, 12774–12777 (2017).
Barbachyn, M. R. et al. U-87947E, a protein quinolone antibacterial agent incorporating a bicyclo[1.1.1]pent-1-yl (BCP) subunit. Bioorg. Med. Chem. Lett. 3, 671–676 (1993).
Westphal, M. V., Wolfstädter, B. T., Plancher, J.-M., Gatfield, J. & Carreira, E. M. Evaluation of tert-butyl isosteres: case studies of physicochemical and pharmacokinetic properties, efficacies, and activities. ChemMedChem 10, 461–469 (2015).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Levterov, V. V. et al. Photochemical in-flow synthesis of 2,4-methanopyrrolidines: pyrrolidine analogues with improved water solubility and reduced lipophilicity. J. Org. Chem. 83, 14350–14361 (2018).
Homon, A. A. et al. 4-(Di-/Trifluoromethyl)-2-heterabicyclo[2.1.1]hexanes: advanced fluorinated phenyl isosteres and proline analogues. Eur. J. Org. Chem. 2021, 6580–6590 (2021).
Wiberg, K. B., Waddell, S. T. & Laidig, K. [1.1.1]Propellane: Reaction with free radicals. Tetrahedron Lett. 27, 1553–1556 (1986).
Kaszynski, P. & Michl, J. A practical photochemical synthesis of bicyclo [1.1.1] pentane-1, 3-dicarboxylic acid. J. Org. Chem. 53, 4593–4594 (1988).
Wiberg, K. B. & Waddell, S. T. Reactions of [1.1.1] propellane. J. Am. Chem. Soc. 112, 2194–2216 (1990).
Bunker, K. D., Sach, N. W., Huang, Q. & Richardson, P. F. Scalable synthesis of 1-bicyclo[1.1.1]pentylamine via a hydrohydrazination reaction. Org. Lett. 13, 4746–4748 (2011).
Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5300 (2018).
Bär, R. M., Kirschner, S., Nieger, M. & Bräse, S. Alkyl and aryl thiol addition to [1.1.1]propellane: scope and limitations of a fast conjugation reaction. Chem. Eur. J. 24, 1373–1382 (2018).
Nugent, J. et al. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 9, 9568–9574 (2019).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Kim, J. H., Ruffoni, A., Al-Faiyz, Y. S. S., Sheikh, N. S. & Leonori, D. Divergent strain-release amino-functionalization of [1.1.1]propellane with electrophilic nitrogen-radicals. Angew. Chem. Int. Ed. 59, 8225–8231 (2020).
Shin, S., Lee, S., Choi, W., Kim, N. & Hong, S. Visible-light-induced 1,3-aminopyridylation of [1.1.1]propellane with N-aminopyridinium salts. Angew. Chem. Int. Ed. 60, 7873–7879 (2021).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Shelp, R. A. & Walsh, P. J. Synthesis of BCP benzylamines from 2-azaallyl anions and [1.1.1]propellane. Angew. Chem. Int. Ed. 57, 15857–15861 (2018).
Hughes, J. M. E., Scarlata, D. A., Chen, A. C. Y., Burch, J. D. & Gleason, J. L. Aminoalkylation of [1.1.1]propellane enables direct access to high-value 3-alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 21, 6800–6804 (2019).
Trongsiriwat, N. et al. Reactions of 2-aryl-1,3-dithianes and [1.1.1]propellane. Angew. Chem. Int. Ed. 58, 13416–13420 (2019).
Yu, S., Jing, C., Noble, A. & Aggarwal, V. K. 1,3-Difunctionalizations of [1.1.1]propellane via 1,2-metallate rearrangements of boronate complexes. Angew. Chem. Int. Ed. 59, 3917–3921 (2020).
Schwärzer, K., Zipse, H., Karaghiosoff, K. & Knochel, P. Highly regioselective addition of allylic zinc halides and various zinc enolates to [1.1.1]propellane. Angew. Chem. Int. Ed. 59, 20235–20241 (2020).
Garlets, Z. J. et al. Enantioselective C–H functionalization of bicyclo[1.1.1]pentanes. Nat. Catal. 3, 351–357 (2020).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
VanHeyst, M. D. et al. Continuous flow-enabled synthesis of bench-stable bicyclo[1.1.1]pentane trifluoroborate salts and their utilization in metallaphotoredox cross-couplings. Org. Lett. 22, 1648–1654 (2020).
Kondo, M. et al. Silaboration of [1.1.1]propellane: a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Ed. 59, 1970–1974 (2020).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Shelp, R. A. et al. Strain-release 2-azaallyl anion addition/borylation of [1.1.1]propellane: synthesis and functionalization of benzylamine bicyclo[1.1.1]pentyl boronates. Chem. Sci. 12, 7066–7072 (2021).
Yu, S. et al. Palladium-catalyzed stagewise strain-release-driven C–C activation of bicyclo[1.1.1]pentanyl alcohols. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202200052
Hoque, M. E., Hassan, M. M. M. & Chattopadhyay, B. Remarkably efficient iridium catalysts for directed C(sp2)–H and C(sp3)–H borylation of diverse classes of substrates. J. Am. Chem. Soc. 143, 5022–5037 (2021).
Ishiyama, T. et al. Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate. J. Am. Chem. Soc. 124, 390–391 (2002).
Hinkes, S. P. A. & Klein, C. D. P. Virtues of volatility: a facile transesterification approach to boronic acids. Org. Lett. 21, 3048–3052 (2019).
Yang, Y. et al. Practical and modular construction of C(sp3)-rich alkyl boron compounds. J. Am. Chem. Soc. 143, 471–480 (2021).
Matteson, D. S. & Kim, G. Y. Asymmetric alkyldifluoroboranes and their use in secondary amine synthesis. Org. Lett. 4, 2153–2155 (2002).
Tian, Z. & Kass, S. R. Carbanions in the gas phase. Chem. Rev. 113, 6986–7010 (2013).
Boller, T. M. et al. Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. Intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc. 127, 14263–14278 (2005).
Zhong, R.-L. & Sakaki, S. sp3 C–H borylation catalyzed by iridium(III) triboryl complex: comprehensive theoretical study of reactivity, regioselectivity, and prediction of excellent ligand. J. Am. Chem. Soc. 141, 9854–9866 (2019).
Larsen, M. A., Wilson, C. V. & Hartwig, J. F. Iridium-catalyzed borylation of primary benzylic C–H bonds without a directing group: scope, mechanism, and origins of selectivity. J. Am. Chem. Soc. 137, 8633–8643 (2015).
Huang, G., Kalek, M., Liao, R.-Z. & Himo, F. Mechanism, reactivity, and selectivity of the iridium-catalyzed C(sp3)-H borylation of chlorosilanes. Chem. Sci. 6, 1735–1746 (2015).
Acknowledgements
This work was supported by the NIGMS of the NIH under R35GM130387. I.F.Y. and J.L.M. gratefully acknowledge the National Science Foundation Graduate Research Fellowship Program and the UC Berkeley Graduate Research Fellowship Program for support under DGE 1752814 and DGE 2146752. We thank H. Celik and A. Lund and UC Berkeley’s NMR facility in the College of Chemistry (CoC-NMR) for spectroscopic assistance, in particular for assistance and advice in acquiring J-resolved spectra. Instruments in the CoC-NMR are supported in part by NIH S10OD024998. We thank K. Durkin and D. Small and UC Berkeley’s Molecular Graphics and Computation Facility (MGCF) for assistance and resources for the computations. The MGCF is supported in part by NIH S10OD023532. We thank the QB3/Chemistry Mass Spectrometry Facility for assistance in obtaining high-resolution mass spectrometry data. We wish to thank T. W. Butcher and E. D. Kalkman for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.F.H., A.M. and D.M.V. conceived and created an initial design of the research. I.F.Y., J.L.M., A.M. and A.D-v. performed the synthetic experiments. I.F.Y. conducted the computational and kinetic studies. J.L.M. conducted the competition studies. I.F.Y., A.M., A.E.P., P.K.M., S.V.R. and D.M.V. selected and prepared the bicyclic reagents used in this study. I.F.Y., J.L.M., A.M., D.M.V. and J.F.H. designed and analysed the experiments and prepared the initial paper. All authors contributed to or approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
A.E.P., P.K.M., S.V.R. and D.M.V. are employees of Enamine, which is a chemical supplier of reagents used in the studies in this paper. All other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Patrick Walsh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Tables 1–3 and Discussion.
Supplementary Data 1
Atomic coordinates of optimized structures from DFT calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, I.F., Manske, J.L., Diéguez-Vázquez, A. et al. Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. Nat. Chem. 15, 685–693 (2023). https://doi.org/10.1038/s41557-023-01159-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01159-4
This article is cited by
-
Programmable late-stage functionalization of bridge-substituted bicyclo[1.1.1]pentane bis-boronates
Nature Chemistry (2024)
-
Iodopentafluorosulfanylation of [1.1.1]propellane and further functionalizations
Science China Chemistry (2023)