Abstract
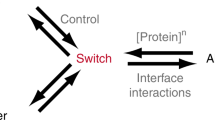
Over half of all the natural nanomachines in living organisms are multimeric and likely exploit the self-assembly of their components to provide functional benefits. However, the advantages and disadvantages of building nanosystems using multiple molecular components remain relatively unexplored at the thermodynamic, kinetic and functional levels. In this study we used theory and a simple DNA-based model that forms the same nanostructures with different numbers of components to advance our knowledge in this area. Despite its lower assembly rate, we found that a system built with three components may undergo a more cooperative assembly transition from less preorganized components, which facilitates the emergence of functionalities. Using simple variations of its components, we also found that trimeric nanosystems display a much higher level of programmability than their dimeric counterparts because they can assemble with various levels of cooperativity, self-inhibition and time-dependent properties. We show here how two simple strategies (for example, cutting and adding components) can be employed to efficiently programme the regulatory function of a more complex, artificially selected, RNA-cleaving catalytic nanosystem.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and the Supplementary Information. The datasets generated and/or analysed during the study are also available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The equations and examples of the codes used for the simulations are described in the Supplementary Information. The MATLAB codes used to perform the numerical simulations of the equilibrium binding experiments and the kinetic traces are also available from the corresponding author upon reasonable request.
References
Grzybowski, B. A. & Huck, W. T. S. The nanotechnology of life-inspired systems. Nat. Nanotechnol. 11, 585–592 (2016).
Murphy, K. G. & Bloom, S. R. Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859 (2006).
Cameron, D. E., Bashor, C. J. & Collins, J. J. A brief history of synthetic biology. Nat. Rev. Microbiol. 12, 381–390 (2014).
Benner, S. A. & Sismour, A. M. Synthetic biology. Nat. Rev. Genet. 6, 533–542 (2005).
Camilli, A. & Bassler, B. L. Bacterial small-molecule signaling pathways. Science 311, 1113–1116 (2006).
Dill, K. A. & MacCallum, J. L. The protein-folding problem, 50 years on. Science 338, 1042–1046 (2012).
Marsh, J. A. & Teichmann, S. A. Structure, dynamics, assembly, and evolution of protein complexes. Annu. Rev. Biochem. 84, 551–575 (2015).
Lynch, M. The evolution of multimeric protein assemblages. Mol. Biol. Evol. 29, 1353–1366 (2012).
Thomas, C. & Lumb, A. B. Physiology of haemoglobin. Continuing Education in Anaesthesia Critical Care & Pain 12, 251–256 (2012).
Pillai, A. S. et al. Origin of complexity in haemoglobin evolution. Nature 581, 480–485 (2020).
Erbas-Cakmak, S. et al. Molecular logic gates: the past, present and future. Chem. Soc. Rev. 47, 2228–2248 (2018).
Galés, C. et al. Real-time monitoring of receptor and G-protein interactions in living cells. Nat. Methods 2, 177–184 (2005).
Pakulska, M. M., Miersch, S. & Shoichet, M. S. Designer protein delivery: from natural to engineered affinity-controlled release systems. Science 351, aac4750 (2016).
Kinch, L. N. & Grishin, N. V. Evolution of protein structures and functions. Curr. Opin. Struct. Biol. 12, 400–408 (2002).
Nooren, I. M. A. & Thornton, J. M. Diversity of protein–protein interactions. EMBO J. 22, 3486–3492 (2003).
Plaxco, K. W., Simons, K. T., Ruczinski, I. & Baker, D. Topology, stability, sequence, and length: defining the determinants of two-state protein folding kinetics. Biochemistry 39, 11177–11183 (2000).
Grantcharova, V., Alm, E. J., Baker, D. & Horwich, A. L. Mechanisms of protein folding. Curr. Opin. Struct. Biol. 11, 70–82 (2001).
Marianayagam, N. J., Sunde, M. & Matthews, J. M. The power of two: protein dimerization in biology. Trends Biochem. Sci. 29, 618–625 (2004).
Whitty, A. Cooperativity and biological complexity. Nat. Chem. Biol. 4, 435–439 (2008).
Williamson, J. R. Cooperativity in macromolecular assembly. Nat. Chem. Biol. 4, 458–465 (2008).
Mattia, E. & Otto, S. Supramolecular systems chemistry. Nat. Nanotechnol. 10, 111–119 (2015).
Seeman, N. C. & Sleiman, H. F. DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017).
Li, M. et al. In vivo production of RNA nanostructures via programmed folding of single-stranded RNAs. Nat. Commun. 9, 2196 (2018).
Gradišar, H. et al. Design of a single-chain polypeptide tetrahedron assembled from coiled-coil segments. Nat. Chem. Biol. 9, 362–366 (2013).
Shekhawat, S. S. & Ghosh, I. Split-protein systems: beyond binary protein–protein interactions. Curr. Opin. Chem. Biol. 15, 789–797 (2011).
Debiais, M., Lelievre, A., Smietana, M. & Müller, S. Splitting aptamers and nucleic acid enzymes for the development of advanced biosensors. Nucleic Acids Res. 48, 3400–3422 (2020).
Dolberg, T. B. et al. Computation-guided optimization of split protein systems. Nat. Chem. Biol. 17, 531–539 (2021).
Michnick, S. W., Ear, P. H., Manderson, E. N., Remy, I. & Stefan, E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat. Rev. Drug Discov. 6, 569–582 (2007).
Cisse, I. I., Kim, H. & Ha, T. A rule of seven in Watson–Crick base-pairing of mismatched sequences. Nat. Struct. Mol. Biol. 19, 623–627 (2012).
Vallée-Bélisle, A., Ricci, F. & Plaxco, K. W. Thermodynamic basis for the optimization of binding-induced biomolecular switches and structure-switching biosensors. Proc. Natl Acad. Sci. USA 106, 13802–13807 (2009).
Hughes, R. A. & Ellington, A. D. Synthetic DNA Synthesis and assembly: putting the synthetic in synthetic biology. Cold Spring Harb. Perspect. Biol. 9, a023812 (2017).
Madsen, M. & Gothelf, K. V. Chemistries for DNA nanotechnology. Chem. Rev. 119, 6384–6458 (2019).
Sadowski, J. P., Calvert, C. R., Zhang, D. Y., Pierce, N. A. & Yin, P. Developmental self-assembly of a DNA tetrahedron. ACS Nano 8, 3251–3259 (2014).
Hao, C. et al. Construction of RNA nanocages by re-engineering the packaging RNA of Phi29 bacteriophage. Nat. Commun. 5, 3890 (2014).
Silverman, S. K. Catalytic DNA: scope, applications, and biochemistry of deoxyribozymes. Trends Biochem. Sci. 41, 595–609 (2016).
Peña, M. D. L., Dufour, D. & Gallego, J. Three-way RNA junctions with remote tertiary contacts: a recurrent and highly versatile fold. RNA 15, 1949–1964 (2009).
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Idili, A., Ricci, F. & Vallée-Bélisle, A. Determining the folding and binding free energy of DNA-based nanodevices and nanoswitches using urea titration curves. Nucleic Acids Res. 45, 7571–7580 (2017).
Lawrence, C. et al. A comparison of the folding kinetics of a small, artificially selected DNA aptamer with those of equivalently simple naturally occurring proteins. Protein Sci. 23, 56–66 (2014).
Zadeh, J. N. et al. NUPACK: analysis and design of nucleic acid systems. J. Comput. Chem. 32, 170–173 (2011).
Jackson, S. E. & Fersht, A. R. Folding of chymotrypsin inhibitor 2. 1. Evidence for a two-state transition. Biochemistry 30, 10428–10435 (1991).
Maxwell, K. L. et al. Protein folding: defining a ‘standard’ set of experimental conditions and a preliminary kinetic data set of two-state proteins. Protein Sci. 14, 602–616 (2005).
Plaxco, K. W., Simons, K. T. & Baker, D. Contact order, transition state placement and the refolding rates of single domain proteins. J. Mol. Biol. 277, 985–994 (1998).
Sanchez-Ruiz, J. M. Protein kinetic stability. Biophys. Chem. 148, 1–15 (2010).
Baskakov, I. V., Legname, G., Prusiner, S. B. & Cohen, F. E. Folding of prion protein to its native α-helical conformation is under kinetic control. J. Biol. Chem. 276, 19687–19690 (2001).
Waudby, C. A., Dobson, C. M. & Christodoulou, J. Nature and regulation of protein folding on the ribosome. Trends Biochem. Sci 44, 914–926 (2019).
Thommen, M., Holtkamp, W. & Rodnina, M. V. Co-translational protein folding: progress and methods. Curr. Opin. Struct. Biol. 42, 83–89 (2017).
Wells, J. A. & McClendon, C. L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 450, 1001–1009 (2007).
Hulme, E. C. & Trevethick, M. A. Ligand binding assays at equilibrium: validation and interpretation. Br. J. Pharmacol. 161, 1219–1237 (2010).
Tian, L. & Heyduk, T. Bivalent ligands with long nanometer-scale flexible linkers. Biochemistry 48, 264–275 (2009).
Lescoute, A. & Westhof, E. Topology of three-way junctions in folded RNAs. RNA 12, 83–93 (2006).
Ercolani, G. & Schiaffino, L. Allosteric, chelate, and interannular cooperativity: a mise au point. Angew. Chem. Int. Ed. 50, 1762–1768 (2011).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Wang, J., Liu, K., Xing, R. & Yan, X. Peptide self-assembly: thermodynamics and kinetics. Chem. Soc. Rev. 45, 5589–5604 (2016).
Li, G. & Zhang, X. C. GTP hydrolysis mechanism of Ras-like GTPases. J. Mol. Biol. 340, 921–932 (2004).
Lu, K. P., Finn, G., Lee, T. H. & Nicholson, L. K. Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 (2007).
Hou, W.-S. & Van Parijs, L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5, 583–589 (2004).
Torabi, S.-F. et al. In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing. Proc. Natl Acad. Sci. USA 112, 5903–5908 (2015).
Maity, T. S. & Weeks, K. M. A threefold RNA–protein interface in the signal recognition particle gates native complex assembly. J. Mol. Biol. 369, 512–524 (2007).
Yi, J., Balagopalan, L., Nguyen, T., McIntire, K. M. & Samelson, L. E. TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps. Nat. Commun. 10, 277 (2019).
Hunter, C. A. & Anderson, H. L. What is cooperativity? Angew. Chem. Int. Ed. 48, 7488–7499 (2009).
Robustelli, P., Piana, S. & Shaw, D. E. Mechanism of coupled folding-upon-binding of an intrinsically disordered protein. J. Am. Chem. Soc. 142, 11092–11101 (2020).
Chen, A., Yan, M. & Yang, S. Split aptamers and their applications in sandwich aptasensors. Trends Analyt. Chem. 80, 581–593 (2016).
Müller, J. & Johnsson, N. Split-ubiquitin and the split-protein sensors: chessman for the endgame. ChemBioChem 9, 2029–2038 (2008).
Shiba, K. & Schimmel, P. Functional assembly of a randomly cleaved protein. Proc. Natl Acad. Sci. USA 89, 1880 (1992).
Cabantous, S. et al. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci. Rep. 3, 2854 (2013).
Rohl, C. A., Strauss, C. E. M., Misura, K. M. S. & Baker, D. in Methods in Enzymology, Vol. 383 66–93 (Academic Press, 2004).
Dagliyan, O. et al. Computational design of chemogenetic and optogenetic split proteins. Nat. Commun. 9, 4042 (2018).
Ricci, F., Vallée-Bélisle, A., Porchetta, A. & Plaxco, K. W. Rational design of allosteric inhibitors and activators using the population-shift model: in vitro validation and application to an artificial biosensor. J. Am. Chem. Soc. 134, 15177–15180 (2012).
Simon, A. J., Vallée-Bélisle, A., Ricci, F. & Plaxco, K. W. Intrinsic disorder as a generalizable strategy for the rational design of highly responsive, allosterically cooperative receptors. Proc. Natl Acad. Sci. USA 111, 15048–15053 (2014).
Simon, A. J., Vallée-Bélisle, A., Ricci, F., Watkins, H. M. & Plaxco, K. W. Using the population-shift mechanism to rationally introduce ‘Hill-type’ cooperativity into a normally non-cooperative receptor. Angew. Chem. Int. Ed. 53, 9471–9475 (2014).
Kang, D., Vallée-Bélisle, A., Porchetta, A., Plaxco, K. W. & Ricci, F. Re-engineering electrochemical biosensors to narrow or extend their useful dynamic range. Angew. Chem. Int. Ed. 51, 6717–6721 (2012).
Porchetta, A., Vallée-Bélisle, A., Plaxco, K. W. & Ricci, F. Allosterically tunable, DNA-based switches triggered by heavy metals. J. Am. Chem. Soc. 135, 13238–13241 (2013).
Harroun, S. G. et al. Programmable DNA switches and their applications. Nanoscale 10, 4607–4641 (2018).
Vallée-Bélisle, A., Ricci, F. & Plaxco, K. W. Engineering biosensors with extended, narrowed, or arbitrarily edited dynamic range. J. Am. Chem. Soc. 134, 2876–2879 (2012).
Ricci, F., Vallée-Bélisle, A., Simon, A. J., Porchetta, A. & Plaxco, K. W. Using nature’s ‘tricks’ to rationally tune the binding properties of biomolecular receptors. Acc. Chem. Res. 49, 1884–1892 (2016).
Lauzon, D., Zhu, G. & Vallée-Bélisle, A. in DNA‐ and RNA‐Based Computing Systems 105–124 (Wiley-VCH, 2021).
Gao, X. J., Chong, L. S., Kim, M. S. & Elowitz, M. B. Programmable protein circuits in living cells. Science 361, 1252–1258 (2018).
Best, J. A., Nijhout, H. F. & Reed, M. C. Homeostatic mechanisms in dopamine synthesis and release: a mathematical model. Theor. Biol. Med. Model. 6, 21 (2009).
Reed, M., Best, J., Golubitsky, M., Stewart, I. & Nijhout, H. F. Analysis of homeostatic mechanisms in biochemical networks. Bull. Math. Biol. 79, 2534–2557 (2017).
Patra, J. K. et al. Nano-based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16, 71 (2018).
Lu, H. et al. Recent progress on nanostructures for drug delivery applications. J. Nanomater. 5762431 (2016).
Pasek, S., Risler, J.-L. & Brézellec, P. Gene fusion/fission is a major contributor to evolution of multi-domain bacterial proteins. Bioinformatics 22, 1418–1423 (2006).
Snel, B., Bork, P. & Huynen, M. Genome evolution: gene fusion versus gene fission. Trends Genet. 16, 9–11 (2000).
Kuriyan, J. & Eisenberg, D. The origin of protein interactions and allostery in colocalization. Nature 450, 983–990 (2007).
Pandey, N., Nobles, C. L., Zechiedrich, L., Maresso, A. W. & Silberg, J. J. Combining random gene fission and rational gene fusion to discover near-infrared fluorescent protein fragments that report on protein–protein interactions. ACS Synth. Biol. 4, 615–624 (2015).
Hagner, K., Setayeshgar, S. & Lynch, M. Stochastic protein multimerization, activity, and fitness. Phys. Rev. E 98, 062401 (2018).
Hashimoto, K., Nishi, H., Bryant, S. & Panchenko, A. R. Caught in self-interaction: evolutionary and functional mechanisms of protein homooligomerization. Phys. Biol. 8, 035007 (2011).
You, Y., Tataurov, A. V. & Owczarzy, R. Measuring thermodynamic details of DNA hybridization using fluorescence. Biopolymers 95, 472–486 (2011).
Galau, G. A., Britten, R. J. & Davidson, E. H. Studies on nucleic acid reassociation kinetics: rate of hybridization of excess RNA with DNA, compared to the rate of DNA renaturation. Proc. Natl Acad. Sci. USA 74, 1020–1023 (1977).
Acknowledgements
This research was conducted through Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (RGPIN-2020-06975, A.V.-B.). A.V.-B. is Canada Research Chair in Bioengineering and Bionanotechnology, Tier II. D.L. acknowledges a Canada graduate scholarship master (CGS M) from NSERC and a third cycle scholarship from the Fonds de recherche du Québec—Nature et technologies (FRQNT). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors would like to thank J. A. Marsh, J. N. Pelletier, L. Pedro, K. Nemčeková and S. G. Harroun for their helpful discussions and comments on the paper. The authors would also like to thank all members of the Quebec Network for Research on Protein Function, Engineering, and Applications (PROTEO) for helpful discussion, more specifically C. R. Landry. The authors would also like to thank the Département de Biochimie et Médecine Moléculaire de l'Université de Montréal for providing us access to their instruments.
Author information
Authors and Affiliations
Contributions
D.L. and A.V.-B. designed the experiments and D.L. performed all the experiments. D.L. and A.V.-B. designed the figures and wrote the paper. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Aleksei Aksimentiev, James Carothers and Guillaume Gines for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Nupack analysis of our DNA based nanostructures.
(a) The 1C system displays a predicted free energy (ΔG°Ass) of −22.94 kcal·mol−1 while its preorganized loops, which cannot be unfolded using urea (Fig. S2), show a predicted ΔG°Ass of −17.25 kcal·mol−1. The subtraction of both leads to a free energy of assembly (ΔG°Ass) of −5.69 kcal·mol−1. (b) The 2C system displays a predicted ΔG°Ass of −25.55 kcal·mol−1 while its preorganized loop has a predicted ΔG°Ass of −8.17 kcal·mol−1. The subtraction of both leads to a ΔG°Ass of −17.38 kcal·mol−1. (c) The 3C system displays a ΔG°Ass of −28.16 kcal·mol−1. (d) All ΔG°Ass predicted by NUPACK are in good agreement with the experimentally derived values and correlate with the number of base pairs involved in the transition. Of note, NUPACK seems to overestimate the ΔG°Ass of the 2C system and the 3C system. This is not unusual as similar discrepancies were observed for the urea denaturation of DNA-DNA complexes38. Data and errors are presented as the values obtained from the non-linear regression of the denaturation experiments (see Fig. S1, Extended Data Fig. 2 and Extended Data Fig. 3) (n = 1).
Extended Data Fig. 2 Thermal denaturation analyses of the 1C, 2C and 3C systems.
(a) Thermal denaturation profiles of the 1-component (black), 2-component (blue) and 3-component systems (green). (b) Left: Van’t Hoff analysis of the thermal denaturation curves enables the extraction of the thermodynamic parameters ΔH°Ass and ΔS°Ass (see panels c) and d), respectively). Right: extrapolation of the ΔG°Ass at 23˚C (right side) are in good agreement with the values obtained using urea denaturation. (c, d) As observed for the m-values and ΔG°Ass (see Fig. S3), ΔH°Ass and ΔS°Ass are also linearly dependant on the number of base pairs broken/formed in the assembly/disassembly transition. ΔH°Ass = −60 ± 1 kcal·mol−1, −85.8 ± 0.8 kcal·mol−1, and −130 ± 7 kcal·mol−1, for the 1C, 2C, and 3C system, respectively. ΔS°Ass = −179 ± 4 cal·mol−1·K−1, −241 ± 3 cal·mol−1·K−1, and −355 ± 22 cal·mol−1·K−1 for the 1C, 2C, and 3C system, respectively. All experiments are performed in PBS buffer (10 mM NaH2PO4, 40 mM NaCl, pH = 7.00). Data and errors are presented as the values obtained from the Van’t Hoff linear regression (n = 1).
Extended Data Fig. 3 Kinetic analysis (chevron plot) of the 1C, 2C, and 3C systems.
The kinetics of the association/folding (circles) and dissociation/unfolding (diamonds) of all nanosystems under various urea concentrations (chevron plot) reveal a two-state association/dissociation mechanism around their denaturation transition (see Table S1). The 2C and 3C systems display similar unfolding transitions (kU,2C = 8.6 ± 3.1 × 10−6 s−1 and kU,3C = 8.3 ± 3.2 × 10−6 s−1; mU,2C = 1.24 ± 0.04 kcal·mol−1·M−1 and mU,3C = 1.41 ± 0.06 kcal·mol−1·M−1) suggesting that they dissociate via the same mechanism (dissociation of a complete strand). In contrast, the 1C system displays an unfolding rate that is 3000-time faster (kU,1C = 2.2 ± 0.9 × 10−2 s−1) with a 3-time smaller urea dependency (mU,1C = 0.43 ± 0.01 kcal·mol−1·M−1). This is consistent with the smaller local unfolding expected to take place for the 1C system, the unfolding slope (mU) is proportional to the surface area made accessible upon dissociation/unfolding. The slower dissociation kinetics of the 2C and 3C systems, extrapolated in absence of urea, is also consistent with the fact their dissociation requires the disruption of 20 base pairs compared to the disruption of 10 base pairs for the 1C system (see cartoon). Interestingly, the dissociation mechanism for the 2C and 3C systems becomes non-linear and similar to the 1C system at a high concentration of urea (>7 M) suggesting that their disassembly becomes only rate limited by the opening of one arm. For raw kinetic traces see Fig. S4 to Fig. S6.
Extended Data Fig. 4 The assembly behaviour of the 2C and 3C systems (Fig. 2) are well predicted by numerical simulations.
Top. Because the one-component system already folds into its active conformation, its activity remains linear with its concentration. Middle. The assembly of the two-components system induced by an increase of component A can be triggered at different [A]50% by increasing the concentration of the limiting component (here B). While [A]50% shifts towards higher concentration, the dynamic range (DR) shifts from 81-fold to 9-fold. Bottom. The assembly of the three-components system induced by an increase of component A can also be triggered at different [A]50% by increasing the concentration of the limiting components (here B and C). Interestingly, the [A]50% shifts from high to low concentration and shifts back to high concentration, while the dynamic range (DR) shifts from 729-fold to 9-fold. Of note, at a low concentration of components B and C, the overall yield of the 3-component system decreases in the presence of large excess of component A. This is because the system favours the formation of the two dimeric intermediates (here AB and AC) instead of the trimer (see also Fig. 3 and Extended Data Fig. 6). Numerical simulations are done using MATLAB® with a dimeric stability of −8 kcal·mol−1 (ΔG°Dim) and a trimeric stability of −16 kcal·mol−1 (ΔG°Tri) leading to an overall assembly stability of −24 kcal·mol−1 (ΔG°Ass = ΔG°Dim + ΔG°Tri).
Extended Data Fig. 5 Effect of the thymine spacer length between each arm on the 3-way junction stability.
(a) Melting curve analysis of the dimeric equilibrium reveals that, as expected, the spacer length (0 T, 2 T, and 4 T) does not substantially change the dimeric affinity (ΔG°Dim). (b) Melting curve analysis of the trimeric equilibrium reveals a strong dependence of the spacer length on the trimeric affinity (ΔG°Tri). *Of note, to measure the trimeric affinity, ΔG°Tri, and to exclude the dimeric energy, we ‘locked’ the dimer into a unimolecular system using a 4 T loop (black loop at the bottom of the blue-green arm). The DNA-hairpin, therefore, mimics the dimeric structure. Inset of panels (a) and (b) represent the derivative of the melting curve (dF/dT) fitted with a Gaussian distribution, which has been used to extract the Tm that are then fitted using Eq. 4 to extract thermodynamic parameters. All experiments have been done in PBS buffer (50 mM Na2HPO4, 400 mM NaCl, pH = 7.00).
Extended Data Fig. 6 Numerical simulations of the effect of destabilizing only ΔG°Tri on the assembly of a 3-component system.
When ΔG°Tri and ΔG°Dim are similar (ΔΔG°Tri-Dim = 0 kcal·mol-1) the assembly of the 3-component system is not favoured (lightest grey). Increasing the value of ΔΔG°Tri-Dim leads to a better yield of assembly of the 3-component system. However, when component A becomes more concentrated than the dissociation constant of the dimeric intermediates (KDim), the assembly of the 3-component system becomes less favourable and the dimeric intermediates AB and AC form instead. A higher ΔΔG°Tri-Dim enables to minimize this effect. Numerical simulations are done using MATLAB®. All ΔG°Dim are fixed at −8 kcal·mol−1 and ΔG°Tri is varied between −8 kcal·mol−1 and −16 kcal·mol−1. Temperature is fixed at 37˚C (310.15 K), the concentration of components B and C are fixed at 100 nM and the concentration of component A is varied from 0.1 nM to 100 µM.
Extended Data Fig. 7 Native PAGE titration.
(a, b) Titrations analyzed by native polyacrylamide gel electrophoresis (native PAGE) support the dimers sequestration mechanism. Like our fluorescence titration experiments (Fig. 3b, right), the 2 T system displays more cooperativity (DR = 12 ± 5) than the 4 T system (DR = 100 ± 47). Furthermore, the 0 T system (black) shows a decrease in assembly when the concentration of component A is larger than the concentration of components B and C (1 μM) (see the reduction of trimer band and increase of dimer band, dimeric intermediates AB and AC). This phenomenon is also observed for the 2 T and 4 T systems, although requiring a much higher concentration of component A. This result is in good agreement with our numerical simulations (see Extended Data Fig. 6) Black = 0 T spacer, Green = 2 T spacer and Blue = 4 T spacer. *Of note, this experiment has been done at room temperature (~ 23 °C) whereas the fluorescence titration has been done at 37 °C. Also, because PAGE is a less sensitive technique compared to fluorescence titration, this experiment has been performed at 1 μM of components B and C compared to the 100 nM used in fluorescence titration. Nonetheless, both experiments demonstrate the same tendencies and are in good agreement with our numerical simulations. Binding curves of 2 T and 4 T spacer are fitted using the Hill equation (Eq. 5) while the binding curve of the 0 T spacer is fitted using a double dose-response curve.
Extended Data Fig. 8 Numerical simulation of the time-dependent assembly of a 3C system.
Using the rate constants measured from Fig. S13 and Fig. S14, we can predict the kinetic traces of assembly of the dimer (plain line) and the trimer (dashed line) using numerical simulation. This enables us to test different concentration conditions before experimentation. (a) Changing [A] only affects dimer assembly while (b) changing [B] and [C] affects the formation of the trimer. This illustrates that the limiting step of trimer assembly, in these conditions, is the formation of the productive dimer BC. These results agree with our experimental data (Fig. 4b in main text and Fig. S16 and Fig. S17). Numerical simulations are performed using MATLAB®.
Extended Data Fig. 9 Adding a third component.
(a) A trimeric assembly can also be created by introducing a new component, called the controller, that can interact with both the native DNAzyme and the substrate. (b) This controller strand can modulate the level of activity and the deactivation time via the formation of an inactive trimer. (c) In the presence of a 10 nM controller and DNAzyme, increasing the concentration of substrate increases the formation of the active dimer and the catalytic rate without substantially affecting the trimeric deactivation rate kTri. (d) At 100 nM substrate, increasing the concentration of controller and DNAzyme increases the rate of trimer formation and thus the rate of DNAzyme deactivation. Kinetic traces (left panel) are fitted using a single exponential (native) or a double exponential (with controller) to extract rate constants of dimeric and trimeric formation (that is, deactivation of the DNAzyme) (Eq. 6). The derivatives (middle panel) are plotted to better show the variation in initial rates and deactivation times. Fluorescence data were converted to the concentration of product using a calibration curve (data not shown) and normalized by the concentration of DNAzyme. All experiments have been done in PBS buffer (50 mM Na2HPO4, 400 mM NaCl, pH = 7.00) at 25 °C.
Supplementary information
Supplementary Information
Chemicals, oligonucleotides synthesis protocol, DNA sequences, 2C and 3C binding curve models, Supplementary Figs. 1–24, MATLAB codes and references.
Supplementary Data 1
Statistical data for Supplementary Figs. 1–24.
Source data
Source Data All Figures
Statistical data for Figs. 1–5 and Extended Data Figs. 1–9.
Source Data Extended Data Fig. 7
Unprocessed gels (0T, 2T and 4T systems).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lauzon, D., Vallée-Bélisle, A. Functional advantages of building nanosystems using multiple molecular components. Nat. Chem. 15, 458–467 (2023). https://doi.org/10.1038/s41557-022-01127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01127-4