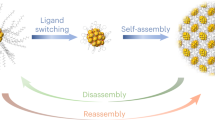
Abstract
The controllable packing of functional nanoparticles (NPs) into crystalline lattices is of interest in the development of NP-based materials. Here we demonstrate that the size, morphology and symmetry of such supercrystals can be tailored by adjusting the surface dynamics of their constituent NPs. In the presence of excess tetraethylammonium cations, atomically precise [Au25(SR)18]− NPs (where SR is a thiolate ligand) can be crystallized into micrometre-sized hexagonal rod-like supercrystals, rather than as face-centred-cubic superlattices otherwise. Experimental characterization supported by theoretical modelling shows that the rod-like crystals consist of polymeric chains in which Au25 NPs are held together by a linear SR–[Au(I)–SR]4 interparticle linker. This linker is formed by conjugation of two dynamically detached SR–[Au(I)–SR]2 protecting motifs from adjacent Au25 particles, and is stabilized by a combination of CH⋯π and ion-pairing interactions between tetraethylammonium cations and SR ligands. The symmetry, morphology and size of the resulting supercrystals can be systematically tuned by changing the concentration and type of the tetraalkylammonium cations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available with the manuscript files and/or from the corresponding authors upon reasonable request. Source data are provided in the Source Data or Supplementary Data files. Extensive DFT and MD calculations have been performed to illustrate the structure of Au25 NP supercrystals, which generated hundreds of raw data files. The results have been summarized in the main and supplementary figures. Due to the large number of raw simulation files, these are not provided with the supporting material but are available from the authors on request. Source data are provided with this paper.
References
Zhang, C. et al. A general approach to DNA-programmable atom equivalents. Nat. Mater. 12, 741–746 (2013).
Shevchenko, E. V., Talapin, D. V., Kotov, N. A., O’Brien, S. & Murray, C. B. Structural diversity in binary nanoparticle superlattices. Nature 439, 55–59 (2006).
Zeng, C., Chen, Y., Kirschbaum, K., Lambright, K. J. & Jin, R. Emergence of hierarchical structural complexities in nanoparticles and their assembly. Science 354, 1580–1584 (2016).
Huang, R.-W. et al. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal–organic framework. Nat. Chem. 9, 689–697 (2017).
Takano, S. & Tsukuda, T. Chemically modified gold/silver superatoms as artificial elements at nanoscale: design principles and synthesis challenges. J. Am. Chem. Soc. 143, 1683–1698 (2021).
Kalsin, A. M. et al. Electrostatic self-assembly of binary nanoparticle crystals with a diamond-like lattice. Science 312, 420–424 (2006).
Boles, M. A., Engel, M. & Talapin, D. V. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 116, 11220–11289 (2016).
De Nardi, M. et al. Gold nanowired: a linear (Au25)n polymer from Au25 molecular clusters. ACS Nano 8, 8505–8512 (2014).
Zhao, M. et al. Ambient chemical fixation of CO2 using a robust Ag27 cluster-based two-dimensional metal–organic framework. Angew. Chem. Int. Ed. 59, 20031–20036 (2020).
Ross, M. B., Ku, J. C., Vaccarezza, V. M., Schatz, G. C. & Mirkin, C. A. Nanoscale form dictates mesoscale function in plasmonic DNA–nanoparticle superlattices. Nat. Nanotechnol. 10, 453–458 (2015).
Huang, J.-H., Wang, Z.-Y., Zang, S.-Q. & Mak, T. C. W. Spontaneous resolution of chiral multi-thiolate-protected Ag30 nanoclusters. ACS Cent. Sci. 6, 1971–1976 (2020).
Chen, T. et al. Crystallization-induced emission enhancement: a novel fluorescent Au–Ag bimetallic nanocluster with precise atomic structure. Sci. Adv. 3, e1700956 (2017).
Bodnarchuk, M. I., Kovalenko, M. V., Heiss, W. & Talapin, D. V. Energetic and entropic contributions to self-assembly of binary nanocrystal superlattices: temperature as the structure-directing factor. J. Am. Chem. Soc. 132, 11967–11977 (2010).
Hossain, S. et al. Understanding and designing one-dimensional assemblies of ligand-protected metal nanoclusters. Mater. Horiz. 7, 796–803 (2020).
Desireddy, A. et al. Ultrastable silver nanoparticles. Nature 501, 399–402 (2013).
Tian, Y. et al. Lattice engineering through nanoparticle–DNA frameworks. Nat. Mater. 15, 654–661 (2016).
Auyeung, E. et al. DNA-mediated nanoparticle crystallization into Wulff polyhedra. Nature 505, 73–77 (2014).
Cao, Y. et al. Reversible isomerization of metal nanoclusters induced by intermolecular interaction. Chem 7, 2227–2244 (2021).
Zheng, K., Fung, V., Yuan, X., Jiang, D.-E. & Xie, J. Real time monitoring of the dynamic intracluster diffusion of single gold atoms into silver nanoclusters. J. Am. Chem. Soc. 141, 18977–18983 (2019).
Salassa, G., Sels, A., Mancin, F. & Bürgi, T. Dynamic nature of thiolate monolayer in Au25(SR)18 nanoclusters. ACS Nano 11, 12609–12614 (2017).
Barrabés, N., Zhang, B. & Bürgi, T. Racemization of chiral Pd2Au36(SC2H4Ph)24: doping increases the flexibility of the cluster surface. J. Am. Chem. Soc. 136, 14361–14364 (2014).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Chakraborty, I. & Pradeep, T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271 (2017).
Li, Y. et al. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature 594, 380–384 (2021).
Zhu, M., Aikens, C. M., Hollander, F. J., Schatz, G. C. & Jin, R. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 130, 5883–5885 (2008).
Lopez-Acevedo, O., Kacprzak, K. A., Akola, J. & Häkkinen, H. Quantum size effects in ambient CO oxidation catalysed by ligand-protected gold clusters. Nat. Chem. 2, 329–334 (2010).
Liao, L. et al. An unprecedented kernel growth mode and layer-number-odevity-dependent properties in gold nanoclusters. Angew. Chem. Int. Ed. 59, 731–734 (2020).
Wu, Z. et al. Unraveling the impact of gold(I)–thiolate motifs on the aggregation-induced emission of gold nanoclusters. Angew. Chem. Int. Ed. 59, 9934–9939 (2020).
Zheng, J., Nicovich, P. R. & Dickson, R. M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 58, 409–431 (2007).
Dolamic, I., Knoppe, S., Dass, A. & Bürgi, T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 3, 798 (2012).
Yao, Q. et al. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 8, 927 (2017).
Yao, Q. et al. Counterion-assisted shaping of nanocluster supracrystals. Angew. Chem. Int. Ed. 54, 184–189 (2015).
Zhang, D. et al. Atomic-resolution transmission electron microscopy of electron beam–sensitive crystalline materials. Science 359, 675–679 (2018).
Liu, L. et al. Imaging defects and their evolution in a metal–organic framework at sub-unit-cell resolution. Nat. Chem. 11, 622–628 (2019).
Heaven, M. W., Dass, A., White, P. S., Holt, K. M. & Murray, R. W. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 130, 3754–3755 (2008).
Matus, M. F., Malola, S., Kinder Bonilla, E., Barngrover, B. M., Aikens, C. M., & Häkkinen, H. A. A topological isomer of the Au25(SR)18− nanocluster. Chem. Commun. 56, 8087–8090 (2020).
Kalenius, E. et al. Experimental confirmation of a topological isomer of the ubiquitous Au25(SR)18 cluster in the gas phase. J. Am. Chem. Soc. 143, 1273–1277 (2021).
Enkovaara, J. et al. Electronic structure calculations with GPAW: a real-space implementation of the projector augmented-wave method. J. Phys. Condens. Matter 22, 253202 (2010).
Wang, Z. & Simoncelli, E. P. Translation insensitive image similarity in complex wavelet domain. In: Proceedings ICASSP ’05. IEEE International Conference on Acoustics, Speech, and Signal Processing 2005, Vol. 2, 573–576 (IEEE, 2005).
Häkkinen, H., Walter, M. & Grönbeck, H. Divide and protect: capping gold nanoclusters with molecular gold−thiolate rings. J. Phys. Chem. B 110, 9927–9931 (2006).
Price, R. C. & Whetten, R. L. Raman spectroscopy of benzenethiolates on nanometer-scale gold clusters. J. Phys. Chem. B 110, 22166–22171 (2006).
Baksi, A., Chakraborty, P., Bhat, S., Natarajan, G. & Pradeep, T. [Au25(SR)18]22−: a noble metal cluster dimer in the gas phase. Chem. Commun. 52, 8397–8400 (2016).
Yuan, P. et al. Solvent-mediated assembly of atom-precise gold–silver nanoclusters to semiconducting one-dimensional materials. Nat. Commun. 11, 2229 (2020).
Lahtinen, T. et al. Covalently linked multimers of gold nanoclusters Au102(p-MBA)44 and Au~250(p-MBA)n. Nanoscale 8, 18665–18674 (2016).
Liu, X. et al. Ag2Au50(PET)36 nanocluster: dimeric assembly of Au25(PET)18 enabled by silver atoms. Angew. Chem. Int. Ed. 59, 13941–13946 (2020).
Wen, Z.-R., Guan, Z.-J., Zhang, Y., Lin, Y.-M. & Wang, Q.-M. [Au7Ag9(dppf)3(CF3CO2)7BF4]n: a linear nanocluster polymer from molecular Au7Ag8 clusters covalently linked by silver atoms. Chem. Commun. 55, 12992–12995 (2019).
Wang, C. et al. Monolayer atomic crystal molecular superlattices. Nature 555, 231 (2018).
Bao, W. et al. Approaching the limits of transparency and conductivity in graphitic materials through lithium intercalation. Nat. Commun. 5, 4224 (2014).
Huang, R.-W. et al. [Cu81(PhS)46(tBuNH2)10(H)32]3+ reveals the coexistence of large planar cores and hemispherical shells in high-nuclearity copper nanoclusters. J. Am. Chem. Soc. 142, 8696–8705 (2020).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Martínez, L., Andrade, R., Birgin, E. G. & Martínez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
GROMACS 2019. https://www.gromacs.org/
Pohjolainen, E., Chen, X., Malola, S., Groenhof, G. & Häkkinen, H. A unified AMBER-compatible molecular mechanics force field for thiolate-protected gold nanoclusters. J. Chem. Theory Comput. 12, 1342–1350 (2016).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinforma. 78, 1950–1958 (2010).
Bayly, C. I., Cieplak, P., Cornell, W. & Kollman, P. A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Singh, U. C. & Kollman, P. A. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 5, 129–145 (1984).
Besler, B. H., Merz, K. M. Jr. & Kollman, P. A. Atomic charges derived from semiempirical methods. J. Comput. Chem. 11, 431–439 (1990).
Frisch, M. J. et al. Gaussian 09, revision E.01 (Gaussian, Inc., Wallingford CT, 2013).
Case, D. A. et al. AMBER 12 (University of California, San Francisco, 2012).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Koivisto, J. et al. Acid–base properties and surface charge distribution of the water-soluble Au102(pMBA)44 nanocluster. J. Phys. Chem. C 120, 10041–10050 (2016).
Miyamoto, S. & Kollman, P. A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Bussi, G. & Parrinello, M. Stochastic thermostats: comparison of local and global schemes. Comput. Phys. Commun. 179, 26–29 (2008).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Chakraborty, P. et al. Exploration of CH⋯π interactions involving the π-system of pseudohalide coligands in metal complexes of a Schiff-base ligand. CrystEngComm 17, 4680–4690 (2015).
Acknowledgements
We acknowledge the financial support of the Ministry of Education, Singapore (Academic Research Grant R-279-000-580-112 (J.X) and R-279-000-538-114 (J.X.)) and the National Natural Science Foundation of China (22071174 (J.X.)). The theoretical work at the University of Jyväskylä was supported by the Academy of Finland (grants 292352 (H.H.), 318905 (H.H.), 319208 (H.H.) and H.H.’s Academy Professorship). The computations were done at the CSC computing centre in Finland and in the FGCI–Finnish Grid and Cloud Infrastructure (persistent identifier, urn:nbn:fi:research-infras-2016072533). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.X. and Y.H. supervised the experimental work. J.X. and Q.Y. conceived the idea and designed the experiment. Q.Y., L.L., M.G. and H.X. carried out the experiments and characterizations. Z.W., T.C. and Y.C. contributed to data interpretation and theory development. H.H. supervised the theoretical and computational work. A.P. performed the image similarity analysis correlating Au25 NP models to the TEM data. S.M. performed DFT computations of the single NPs and linked NP models. M.F.M. performed the MD simulations of the linked NP models in aqueous solution. All authors contributed to manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Thalappil Pradeep and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–99, Notes 1–9 and Table 1.
Supplementary Data 1
Source data for Supplementary Figs. 1, 2, 3, 5, 7, 8, 10, 14, 16, 18, 19, 21.
Supplementary Data 2
Source data for Supplementary Figs. 73, 77, 78, 79, 80, 81, 82.
Supplementary Data 3
Source data for Supplementary Figs. 89, 90, 91, 92, 93, 94, 95, 97, 98.
Source data
Source Data Fig. 1
Plottable source data for Fig. 1c,d,f,g.
Source Data Fig. 4
Plottable source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Q., Liu, L., Malola, S. et al. Supercrystal engineering of atomically precise gold nanoparticles promoted by surface dynamics. Nat. Chem. 15, 230–239 (2023). https://doi.org/10.1038/s41557-022-01079-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01079-9
This article is cited by
-
Understanding ligand-protected noble metal nanoclusters at work
Nature Reviews Materials (2023)
-
Luminescence modulation of ultrasmall gold clusters by aromatic ligands
Nano Research (2023)