Abstract
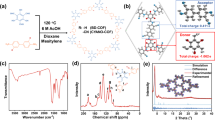
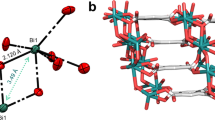
Organic room-temperature phosphorescence, a spin-forbidden radiative process, has emerged as an interesting but rare phenomenon with multiple potential applications in optoelectronic devices, biosensing and anticounterfeiting. Covalent organic frameworks (COFs) with accessible nanoscale porosity and precisely engineered topology can offer unique benefits in the design of phosphorescent materials, but these are presently unexplored. Here, we report an approach of covalent doping, whereby a COF is synthesized by copolymerization of halogenated and unsubstituted phenyldiboronic acids, allowing for random distribution of functionalized units at varying ratios, yielding highly phosphorescent COFs. Such controlled halogen doping enhances the intersystem crossing while minimizing triplet–triplet annihilation by diluting the phosphors. The rigidity of the COF suppresses vibrational relaxation and allows a high phosphorescence quantum yield (ΦPhos ≤ 29%) at room temperature. The permanent porosity of the COFs and the combination of the singlet and triplet emitting channels enable a highly efficient COF-based oxygen sensor, with an ultra-wide dynamic detection range (~103–10−5 torr of partial oxygen pressure).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files and also from the corresponding authors upon reasonable request. Supplementary crystallographic information files have been deposited with the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers ClPBA: CCDC 2119959 and BrPBA: CCDC 2119960. They can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Côté, A. P. et al. Crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Huang, N., Wang, P. & Jiang, D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Keller, N. & Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 50, 1813–1845 (2021).
Jadhav, T. et al. 2D poly (arylene vinylene) covalent organic frameworks via aldol condensation of trimethyltriazine. Angew. Chem. Int. Ed. 58, 13753–13757 (2019).
Dalapati, S., Jin, E., Addicoat, M., Heine, T. & Jiang, D. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 138, 5797–5800 (2016).
Evans, A. M. et al. Emissive single-crystalline boroxine-linked colloidal covalent organic frameworks. J. Am. Chem. Soc. 141, 19728–19735 (2019).
Ding, S.-Y. et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury (II). J. Am. Chem. Soc. 138, 3031–3037 (2016).
Wang, S. et al. Covalent organic frameworks: a platform for the experimental establishment of the influence of intermolecular distance on phosphorescence. J. Mater. Chem. C 6, 5369–5374 (2018).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Kenry, Chen, C. & Liu, B. Enhancing the performance of pure organic room-temperature phosphorescent luminophores. Nat. Commun. 10, 2111 (2019).
Bolton, O., Lee, K., Kim, H. J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3, 205–210 (2011).
An, Z. et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 14, 685–690 (2015).
Yang, J. et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 9, 840 (2018).
Hamzehpoor, E. & Perepichka, D. F. Crystal engineering of room temperature phosphorescence in organic solids. Angew. Chem. Int. Ed. 59, 9977–9981 (2020).
Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539–1544 (2021).
Gu, M. et al. Polymorphism-dependent dynamic ultralong organic phosphorescence. Research 2020, 8183450 (2020).
Lee, J. et al. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. Nat. Mater. 15, 92–98 (2016).
Yum, J.-H. et al. Phosphorescent energy relay dye for improved light harvesting response in liquid dye-sensitized solar cells. Energy Environ. Sci. 3, 434–437 (2010).
Tabachnyk, M., Ehrler, B., Bayliss, S., Friend, R. H. & Greenham, N. C. Triplet diffusion in singlet exciton fission sensitized pentacene solar cells. Appl. Phys. Lett. 103, 190–191 (2013).
Wan, S., Zhou, H., Lin, J. & Lu, W. A prototype of a volumetric three‐dimensional display based on programmable photo‐activated phosphorescence. Angew. Chem. Int. Ed. 132, 8494–8498 (2020).
Filidou, V. et al. Ultrafast entangling gates between nuclear spins using photoexcited triplet states. Nat. Phys. 8, 596–600 (2012).
Wu, Z. et al. Persistent room temperature phosphorescence from triarylboranes: a combined experimental and theoretical study. Angew. Chem. Int. Ed. 132, 17285–17292 (2020).
Hackney, H. E. & Perepichka, D. F. Recent advances in room temperature phosphorescence of crystalline boron containing organic compounds. Aggregate 3, e123 (2022).
Yuan, W. Z. et al. Crystallization-induced phosphorescence of pure organic luminogens at room temperature. J. Phys. Chem. C 114, 6090–6099 (2010).
Yan, X. et al. Recent advances on host-guest material systems toward organic room temperature phosphorescence. Small 18, 2104073 (2022).
Chen, C. et al. Carbazole isomers induce ultralong organic phosphorescence. Nat. Mater. 20, 175–180 (2021).
Nangia, A. K. & Desiraju, G. R. Crystal engineering: an outlook for the future. Angew. Chem. Int. Ed. 58, 4100–4107 (2019).
Deng, H. et al. Multiple functional groups of varying ratios in metal-organic frameworks. Science 327, 846–850 (2010).
Qin, J.-S., Yuan, S., Wang, Q., Alsalme, A. & Zhou, H.-C. Mixed-linker strategy for the construction of multifunctional metal-organic frameworks. J. Mater. Chem. A 5, 4280–4291 (2017).
Yeung, H. H. M. et al. Ligand‐directed control over crystal structures of inorganic-organic frameworks and formation of solid solutions. Angew. Chem. Int. Ed. 52, 5544–5547 (2013).
Li, R. L. et al. Two-dimensional covalent organic framework solid solutions. J. Am. Chem. Soc. 143, 7081–7087 (2021).
Lakshmi, V. et al. A two-dimensional poly (azatriangulene) covalent organic framework with semiconducting and paramagnetic states. J. Am. Chem. Soc. 142, 2155–2160 (2020).
Jin, E. et al. Two-dimensional sp2 carbon-conjugated covalent organic frameworks. Science 357, 673–676 (2017).
Rotter, J. M. et al. Highly conducting Wurster-type twisted covalent organic frameworks. Chem. Sci. 11, 12843–12853 (2020).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48, 5439–5442 (2009).
Kuno, S., Kanamori, T., Yijing, Z., Ohtani, H. & Yuasa, H. Long persistent phosphorescence of crystalline phenylboronic acid derivatives: photophysics and a mechanistic study. ChemPhotoChem 1, 102–106 (2017).
Hamzehpoor, E. et al. Room temperature phosphorescence vs triplet-triplet annihilation in N-substituted acridone solids. J. Phys. Chem. Lett. 12, 6431–6438 (2021).
Tokunaga, Y., Ueno, H. & Shimomura, Y. Formation of boroxine: its stability and thermodynamic parameters in solution. Heterocycles 57, 787–790 (2002).
Tilford, R. W., Mugavero, S. J. III, Pellechia, P. J. & Lavigne, J. J. Tailoring microporosity in covalent organic frameworks. Adv. Mater. 20, 2741–2746 (2008).
Gao, J. & Jiang, D. Covalent organic frameworks with spatially confined guest molecules in nanochannels and their impacts on crystalline structures. Chem. Commun. 52, 1498–1500 (2016).
Chai, Z. et al. Abnormal room temperature phosphorescence of purely organic boron-containing compounds: the relationship between the emissive behavior and the molecular packing, and the potential related applications. Chem. Sci. 8, 8336–8344 (2017).
Wang, X.-D. & Wolfbeis, O. S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 43, 3666–3761 (2014).
Han, B.-H., Manners, I. & Winnik, M. A. Oxygen sensors based on mesoporous silica particles on layer-by-layer self-assembled films. Chem. Mater. 17, 3160–3171 (2005).
Carraway, E., Demas, J. & DeGraff, B. Photophysics and oxygen quenching of transition-metal complexes on fumed silica. Langmuir 7, 2991–2998 (1991).
Barrett, S. M., Wang, C. & Lin, W. Oxygen sensing via phosphorescence quenching of doped metal-organic frameworks. J. Mater. Chem. 22, 10329–10334 (2012).
Liu, S. Y. et al. Flexible, luminescent metal-organic frameworks showing synergistic solid‐solution effects on porosity and sensitivity. Angew. Chem. Int. Ed. 55, 16021–16025 (2016).
Ye, J.-W. et al. Mixed-lanthanide porous coordination polymers showing range-tunable ratiometric luminescence for O2 sensing. lnorg. Chem. 56, 4238–4243 (2017).
Qi, X.-L. et al. Phosphorescence doping in a flexible ultramicroporous framework for high and tunable oxygen sensing efficiency. Chem. Commun. 49, 6864–6866 (2013).
Xie, Z., Ma, L., deKrafft, K. E., Jin, A. & Lin, W. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 132, 922–923 (2010).
Cai, S. et al. Hydrogen‐bonded organic aromatic frameworks for ultralong phosphorescence by intralayer π-π interactions. Angew. Chem. Int. Ed. 57, 4005–4009 (2018).
Hirata, S. et al. Efficient persistent room temperature phosphorescence in organic amorphous materials under ambient conditions. Adv. Funct. Mater. 23, 3386–3397 (2013).
Lehner, P., Staudinger, C., Borisov, S. M. & Klimant, I. Ultra-sensitive optical oxygen sensors for characterization of nearly anoxic systems. Nat. Commun. 5, 4460 (2014).
Cai, S. et al. Enabling long-lived organic room temperature phosphorescence in polymers by subunit interlocking. Nat. Commun. 10, 4247 (2019).
Yu, Y. et al. Room‐temperature‐phosphorescence‐based dissolved oxygen detection by core‐shell polymer nanoparticles containing metal‐free organic phosphors. Angew. Chem. Int. Ed. 56, 16207–16211 (2017).
Zhang, G. et al. Multi-emissive difluoroboron dibenzoylmethane polylactide exhibiting intense fluorescence and oxygen-sensitive room-temperature phosphorescence. J. Am. Chem. Soc. 129, 8942–8943 (2007).
Feng, Y., Cheng, J., Zhou, L., Zhou, X. & Xiang, H. Ratiometric optical oxygen sensing: a review in respect of material design. Analyst 137, 4885–4901 (2012).
Mamlouk, H. et al. Highly active, separable and recyclable bipyridine iridium catalysts for C-H borylation reactions. Catal. Sci. Technol. 8, 124–127 (2018).
Akita, M., Saito, S., Osaka, I., Koganezawac, T. & Takimiya, T. Amide-bridged terphenyl and dithienylbenzene units for semiconducting polymers. RSC Adv. 6, 16437–16447 (2016).
Acknowledgements
This work is supported by a US Army Office of Scientific Research Single Investigator Grant (D.F.P.) and an NSERC of Canada Discovery Grant (D.F.P.). E.H. and C.R. acknowledge FRQNT doctoral scholarships. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank R. Stein (McGill University) for assistance with solid-state NMR spectroscopy, T. Maris (University de Montreal) for X-ray crystallography and A. Jonderian and E. McCalla (McGill University) for access to PXRD measurements. The computational part of this work was enabled, in part, by The Digital Research Alliance of Canada’s compute clusters (https://alliancecan.ca). We are grateful to the Materials Characterization facilities in the Department of Chemistry of McGill University (MC2) and the McGill Institute for Advanced Materials (MIAM) and the Facility of Electron Microscopy Research at McGill.
Author information
Authors and Affiliations
Contributions
E.H. and D.F.P. conceived the project and wrote the Paper. E.H. and Y.T. prepared and characterized the starting materials and COFs. E.H. and C.R. conducted the photoluminescence measurements. C.-H.L. performed the TEM and HAADF mapping. H.M.T. determined the BrPBA crystal structure and performed thermal analysis and Hirshfeld surface analysis. All authors contributed to data analyses and provided comments on the paper. Correspondence and requests for materials should be addressed to D.F.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Florian Auras and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, Discussion and Tables 1–14.
Supplementary Data 1
Source data for Supplementary figures.
Supplementary Data 2
Crystallographic data for ClPBA; CCDC reference 2119959.
Supplementary Data 3
Crystallographic data for BrPBA; CCDC reference 2119960.
Source data
Source Data Fig. 2
Numerical data for panels a,b,c,e,f,h.
Source Data Fig. 3
Numerical data for panels a,b,d,e,f.
Source Data Fig. 4
Numerical data for all panels.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamzehpoor, E., Ruchlin, C., Tao, Y. et al. Efficient room-temperature phosphorescence of covalent organic frameworks through covalent halogen doping. Nat. Chem. 15, 83–90 (2023). https://doi.org/10.1038/s41557-022-01070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01070-4
This article is cited by
-
Isostructural doping for organic persistent mechanoluminescence
Nature Communications (2024)
-
Ultra-fast supercritically solvothermal polymerization for large single-crystalline covalent organic frameworks
Nature Protocols (2024)
-
Water-stable boroxine structure with dynamic covalent bonds
Nature Communications (2024)
-
Delayed room temperature phosphorescence enabled by phosphines
Nature Communications (2024)
-
Room-temperature phosphorescent materials derived from natural resources
Nature Reviews Chemistry (2023)