Abstract
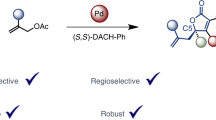
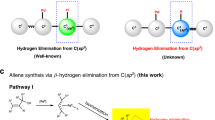
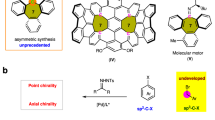
Butafulvene is a constitutional isomer of benzene, comprising a cyclobutene skeleton bearing two exocyclic conjugated methylene units. As a result of the intrinsic high strain energy and anti-aromaticity, the preparation of butafulvene compounds has been a fundamental issue for the development of butafulvene chemistry. Here an efficient palladium-catalysed coupling protocol involving propargylic compounds has been developed, providing a solid and versatile strategy for the rapid assembly of symmetric butafulvene derivatives. Based on mechanistic studies, two complementary mechanisms, both involving palladium catalysis, have been confirmed. With the mechanism unveiled, the synthesis of non-symmetric butafulvenes has also been achieved. Advantages of this strategy include tolerance to a wide range of propargylic molecules, mild reaction conditions, simple catalytic systems and easy scalability. The synthetic potential of the products as platform molecules for cyclobutene derivatives has also been demonstrated.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC1875931 (2a), 2035320 (5h), 2045841 (18a), 2049400 (19a) and 2131557 (22). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Wang, M. & Shi, Z. Methodologies and strategies for selective borylation of C–Het and C–C bonds. Chem. Rev. 120, 7348–7398 (2020).
Qiu, Z. & Li, C.-J. Transformations of less-activated phenols and phenol derivatives via C–O cleavage. Chem. Rev. 120, 10454–10515 (2020).
Evano, G. & Theunissen, C. Beyond Friedel and Crafts: directed alkylation of C–H bonds in arenes. Angew. Chem. Int. Ed. 58, 7202–7236 (2019).
Wertjes, W. C., Southgate, E. H. & Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 47, 7996–8017 (2018).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
You, S.-L. Asymmetric Dearomatization Reactions (Wiley, 2016).
Raviola, C., Protti, S., Ravelli, D. & Fagnoni, M. (Hetero)aromatics from dienynes, enediynes and enyne-allenes. Chem. Soc. Rev. 45, 4364–4390 (2016).
Mortier, J. Arene Chemistry (Wiley, 2015).
Li, L., Mu, X., Liu, W., Mi, Z. & Li, C.-J. Simple and efficient system for combined solar energy harvesting and reversible hydrogen storage. J. Am. Chem. Soc. 137, 7576–7579 (2015).
Chinchilla, R. & Nájera, C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 114, 1783–1826 (2014).
Wang, D.-S., Chen, Q.-A., Lu, S.-M. & Zhou, Y.-G. Asymmetric hydrogenation of heteroarenes and arenes. Chem. Rev. 112, 2557–2590 (2012).
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Ackermann, L. Modern Arylation Methods (Wiley, 2009).
Saito, S. & Yamamoto, Y. Recent advances in the transition-metal-catalyzed regioselective approaches to polysubstituted benzene derivatives. Chem. Rev. 100, 2901–2915 (2000).
Scott, A. P., Agranat, I., Biedermann, P. U., Riggs, N. V. & Radom, L. Fulvalenes, fulvenes and related molecules: an ab initio study. J. Org. Chem. 62, 2026–2038 (1997).
Neuenschwander, M. Substituent effects on π-bond delocalization of fulvenes and fulvalenes. Are fulvenes aromatic? Helv. Chim. Acta 98, 763–784 (2015).
Neuenschwander, M. Low-temperature olefin syntheses in view of parent fulvenes and fulvalenes. Helv. Chim. Acta 98, 731–762 (2015).
Preethalayam, P. et al. Recent advances in the chemistry of pentafulvenes. Chem. Rev. 117, 3930–3989 (2017).
Beckhaus, R. Pentafulvene complexes of group four metals: versatile organometallic building blocks. Coord. Chem. Rev. 376, 467–477 (2018).
Allen, A. D. & Tidwell, T. T. Antiaromaticity in open-shell cyclopropenyl to cycloheptatrienyl cations, anions, free radicals and radical ions. Chem. Rev. 101, 1333–1348 (2001).
Wiberg, K. B. Antiaromaticity in monocyclic conjugated carbon rings. Chem. Rev. 101, 1317–1331 (2001).
Toda, F. & Garratt, P. Four-membered ring compounds containing bis(methylene)cyclobutene or tetrakis(methylene)cyclobutane moieties. Benzocyclobutadiene, benzodicyclobutadiene, biphenylene and related compounds. Chem. Rev. 92, 1685–1707 (1992).
Dong, Y. et al. Aggregation-induced and crystallization-enhanced emissions of 1,2-diphenyl-3,4-bis(diphenylmethylene)-1-cyclobutene. Chem. Commun. 31, 3255–3257 (2007).
Bernstein, H. I. & Quimby, W. C. The photochemical dimerization of trans-cinnamic acid. J. Am. Chem. Soc. 65, 1845–1846 (1943).
Blomquist, A. T. & Meinwald, Y. C. Synthesis of some conjugated cyclobutane polyolefins and their 1,2-cycloaddition to tetracyanoethylene. J. Am. Chem. Soc. 81, 667–672 (1959).
Huntsman, W. D. & Wristers, H. J. 3,4-Dimethylenecyclobutene by thermal rearrangement of 1,5-hexadiyne. J. Am. Chem. Soc. 85, 3308–3309 (1963).
Pasto, D. J. & Yang, S. H. A study of the stereochemistry of the electrocyclic ring closure of substituted bisallenes to substituted 3,4-bisalkylidenecyclobutenes. J. Org. Chem. 54, 3544–3549 (1989).
Huntsman, W. D. & Wristers, H. J. Thermal rearrangement of 1,5-hexadiyne and related compounds. J. Am. Chem. Soc. 89, 342–347 (1967).
Alcaide, B., Almendros, P. & Aragoncillo, C. Exploiting [2 + 2] cycloaddition chemistry: achievements with allenes. Chem. Soc. Rev. 39, 783–816 (2010).
Alcaide, B., Almendros, P. & Aragoncillo, C. Cyclization reactions of bis(allenes) for the synthesis of polycarbo(hetero)cycles. Chem. Soc. Rev. 43, 3106–3135 (2014).
Kitagaki, S., Inagaki, F. & Mukai, C. [2 + 2 + 1] cyclization of allenes. Chem. Soc. Rev. 43, 2956–2978 (2014).
López, F. & Mascareñas, J. L. [4 + 2] and [4 + 3] catalytic cycloadditions of allenes. Chem. Soc. Rev. 43, 2904–2915 (2014).
Lledó, A., Pla-Quintana, A. & Roglans, A. Allenes, versatile unsaturated motifs in transition-metal-catalysed [2 + 2 + 2] cycloaddition reactions. Chem. Soc. Rev. 45, 2010–2023 (2016).
Mascareñas, J. L., Varela, I. & López, F. Allenes and derivatives in gold(I)- and platinum(II)-catalyzed formal cycloadditions. Acc. Chem. Res. 52, 465–479 (2019).
Pasto, D. J. & Mitra, D. K. Synthesis of 3,4-bis(alkylidene)cyclobutenes by the reductive dimerization of propargyl chlorides. J. Org. Chem. 47, 1381–1382 (1982).
Pasto, D. J. & Huang, N.-Z. Electrocyclization and cyclooligomerization reactions of 2,7-dimethyl-2,3,5,6-octatetraene with Ni(0) and Ni(II) complexes. J. Org. Chem. 50, 4465–4467 (1985).
Ito, H., Sasaki, Y. & Sawamura, M. Copper(I)-catalyzed substitution of propargylic carbonates with diboron: selective synthesis of multisubstituted allenylboronates. J. Am. Chem. Soc. 130, 15774–15775 (2008).
Zhao, T. S., Yang, Y., Lessing, T. & Szabo, K. J. Borylation of propargylic substrates by bimetallic catalysis. Synthesis of allenyl, propargylic and butadienyl Bpin derivatives. J. Am. Chem. Soc. 136, 7563–7566 (2014).
Zhao, J. & Szabó, K. J. Catalytic borylative opening of propargyl cyclopropane, epoxide, aziridine and oxetane substrates: ligand controlled synthesis of allenyl boronates and alkenyl diboronates. Angew. Chem. Int. Ed. 55, 1502–1506 (2016).
Mao, L., Szabo, K. J. & Marder, T. B. Synthesis of benzyl-, allyl- and allenyl-boronates via copper-catalyzed borylation of alcohols. Org. Lett. 19, 1204–1207 (2017).
Lü, B. et al. 2,6-Diisopropoxyphenyl(dicyclohexyl)phosphine: a new ligand for palladium-catalyzed amination reactions of aryl chlorides with potassium hydroxide as the base. Adv. Synth. Catal. 353, 100–112 (2011).
Lü, B., Fu, C. & Ma, S. Application of dicyclohexyl-(S)-trimethoxyphenyl phosphine⋅HBF4 salt for the highly selective Suzuki coupling of the C–Cl bond in β-chlorobutenolides over the more reactive allylic C–O bond. Chem. Eur. J. 16, 6434–6437 (2010).
Li, P., Lü, B., Fu, C. & Ma, S. Zheda-Phos for general α-monoarylation of acetone with aryl chlorides. Adv. Synth. Catal. 355, 1255–1259 (2013).
Li, Q., Fu, C. & Ma, S. Catalytic asymmetric allenylation of malonates with the generation of central chirality. Angew. Chem. Int. Ed. 51, 11783–11786 (2012).
Tsutsumi, K., Ogoshi, S., Kakiuchi, K., Nishiguchi, S. & Kurosawa, H. Cross-coupling reactions proceeding through η1- and η3-propargyl/allenyl-palladium(II) intermediates. Inorg. Chim. Acta 296, 37–44 (1999).
Lin, M.-J. & Loh, T.-P. Indium-mediated reaction of trialkylsilyl propargyl bromide with aldehydes: highly regioselective synthesis of allenic and homopropargylic alcohols. J. Am. Chem. Soc. 125, 13042–13043 (2003).
Krause, N. & Hashmi, A. S. K. Modern Allene Chemistry (Wiley, 2004).
Lee, P. H. & Lee, K. Intermolecular tandem Pd-catalyzed cross-coupling/[4 + 4] and [4 + 2] cycloadditions: a one-pot, five-component assembly of bicyclo[6.4.0]dodecanes. Angew. Chem. Int. Ed. 44, 3253–3256 (2005).
Xu, B., Mashuta, M. S. & Hammond, G. B. Crystallographic characterization of difluoropropargyl indium bromide, a reactive fluoroorganometallic reagent. Angew. Chem. Int. Ed. 45, 7265–7267 (2006).
Lee, P. H., Lee, K. & Kang, Y. In situ generation of vinyl allenes and its applications to one-pot assembly of cyclohexene, cyclooctadiene, 3,7-nonadienone, and bicyclo[6.4.0]dodecene derivatives with palladium-catalyzed multicomponent reactions. J. Am. Chem. Soc. 128, 1139–1146 (2006).
Lee, P. H. Indium and gallium-mediated addition reactions. Bull. Korean Chem. Soc. 28, 17–28 (2007).
Zhu, C., Zhang, X., Lian, X. & Ma, S. One-pot approach to installing eight-membered rings onto indoles. Angew. Chem. Int. Ed. 51, 7817–7820 (2012).
Toda, F., Kumada, K., Ishiguro, N. & Akagi, K. Preparation, lithium aluminum hydride reduction, and electronic spectra of halogen-substituted 3,4-bis(diphenylmethylene)cyclobutenes and -cyclobutanes. Bull. Chem. Soc. Jap. 43, 3535–3539 (1970).
Cai, B.-Z. & Blackburn, G. M. The syntheses and reactions of 3,4-bisphosphono-1,2,4,5-tetraenes. Synth. Commun. 27, 3943–3949 (1997).
Delas, C., Urabeb, H. & Satoa, F. Titanium-mediated intramolecular cyclization of tethered propargyl alcohol derivatives. Access to exocyclic bis-allenes and cyclobutene derivatives. Tetrahedron Lett. 42, 4147–4150 (2001).
Parkhurst, R. R. & Swager, T. M. Synthesis of 3,4-bis(benzylidene)cyclobutenes. Synlett 11, 1519–1522 (2011).
Tsuji, J. & Mandai, T. Palladium-catalyzed reactions of propargylic compounds in organic synthesis. Angew. Chem. Int. Ed. 34, 2589–2612 (1995).
Guo, L.-N., Duan, Xin-Hua & Liang, Y.-M. Palladium-catalyzed cyclization of propargylic compounds. Acc. Chem. Res. 44, 111–122 (2011).
Suzuki, A. Organoboron compounds in new synthetic reactions. Pure Appl. Chem. 57, 1749–1758 (1985).
Tyson, E. L., Ament, M. S. & Yoon, T. P. Transition metal photoredox catalysis of radical thiol-ene reactions. J. Org. Chem. 78, 2046–2050 (2013).
Acknowledgements
We acknowledge financial support from the National Key R&D Program of China (2021YFF0701600 for J.Z.), the National Natural Science Foundation of China (22071239 for Q.-A.C. and 21988101 for S.M.), the Dalian Institute of Chemical Physics (DICPI201902 for Q.-A.C.). S.M. is a Qiu Shi Adjunct Professor at Zhejiang University. We thank F. Jiang in our group for reproducing the results of 2o and 12b.
Author information
Authors and Affiliations
Contributions
S.M. and Q.-A.C. conceived and supervised the project. S.M., Q.-A.C., J.Z., X.H. and B.-Z.C. designed the experiments. X.H., B.-Z.C., P.L., D.-W.J., J.L., H.Z., S.-N.Y., Y.-C.H., B.W., X.-P.H., C.F., Y.H. and J.Z. performed the experiments and analysed the data. All authors discussed the results and commented on the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Dorian Didier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General information; synthesis of starting materials; synthesis of symmetric butafulvenes; mechanistic studies; synthesis of non-symmetric butafulvenes; DFT computations; synthetic applications; X-ray crystal structures for 2a, 5h, 18a, 19a and 22; references; copies of the 1H NMR and 13C NMR spectra.
Supplementary Data 1
Crystallographic data for compound 2a; CCDC reference 1875931.
Supplementary Data 2
Crystallographic data for compound 5h; CCDC reference 2035320.
Supplementary Data 3
Crystallographic data for compound 18a; CCDC reference 2045841.
Supplementary Data 4
Crystallographic data for compound 19a; CCDC reference 2049400.
Supplementary Data 5
Crystallographic data for compound 22; CCDC reference 2131557.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Chen, BZ., Li, P. et al. Palladium-catalysed construction of butafulvenes. Nat. Chem. 14, 1185–1192 (2022). https://doi.org/10.1038/s41557-022-01017-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01017-9
This article is cited by
-
Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives
Nature (2023)
-
Nickel-catalyzed divergent Mizoroki–Heck reaction of 1,3-dienes
Nature Communications (2023)