Abstract
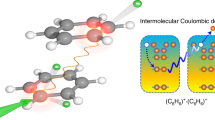
Intermolecular Coulombic decay (ICD) is a process whereby photoexcited molecules relax by ionizing their neighbouring molecules. ICD is efficient when intermolecular interactions are active and consequently it is observed only in weakly bound systems, such as clusters and hydrogen-bonded systems. Here we report an efficient ICD between unbound molecules excited at ambient-light intensities. On the photoexcitation of gas-phase pyridine monomers, well below the ionization threshold and at low laser intensities, we detected the parent and heavier-than-parent cations. The isotropic emission of slow electrons revealed ICD as the underlying process. π–π* excitation in unbounded pyridine monomers triggered an associative interaction between them, which leads to an efficient three-centre ICD. The cation resulting from the molecular association of the three pyridine centres relaxed through fragmentation. This below-threshold ionization under ambient light has implications for the understanding of radiation damage and astrochemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Cederbaum, L. S., Zobeley, J. & Tarantelli, F. Giant intermolecular decay and fragmentation of clusters. Phys. Rev. Lett. 79, 4778–4781 (1997).
Kuleff, A. I., Gokhberg, K., Kopelke, S. & Cederbaum, L. S. Ultrafast interatomic electronic decay in multiply excited clusters. Phys. Rev. Lett. 105, 043004 (2010).
Demekhin, P. V. et al. Overcoming blockade in producing doubly excited dimers by a single intense pulse and their decay. J. Phys. B 46, 021001 (2013).
Jahnke, T. et al. Interatomic and intermolecular coulombic decay. Chem. Rev. 120, 11295–11369 (2020).
Jahnke, T. Interatomic and intermolecular coulombic decay: the coming of age story. J. Phys. B 48, 082001 (2015).
Jahnke, T. et al. Photoelectron and ICD electron angular distributions from fixed-in-space neon dimers. J. Phys. B 40, 2597–2606 (2007).
Ren, X. et al. Ultrafast energy transfer between π-stacked aromatic rings upon inner-valence ionization. Nat. Chem. https://doi.org/10.1038/s41557-021-00838-4 (2022).
Buchta, D. et al. Charge transfer and Penning ionization of dopants in or on helium nanodroplets exposed to EUV radiation. J. Phys. Chem. A 117, 4394–4403 (2013).
Nagaya, K. et al. Interatomic coulombic decay cascades in multiply excited neon clusters. Nat. Commun. 7, 13477 (2016).
Dubrouil, A. et al. Two-photon resonant excitation of interatomic coulombic decay in neon dimers. J. Phys. B 48, 204005 (2015).
LaForge, A. et al. Collective autoionization in multiply-excited systems: a novel ionization process observed in helium nanodroplets. Sci. Rep. 4, 3621 (2014).
Jahnke, T. et al. Ultrafast energy transfer between water molecules. Nat. Phys. 6, 139–142 (2010).
Richter, C. et al. Competition between proton transfer and intermolecular coulombic decay in water. Nat. Commun. 9, 4988 (2018).
Sisourat, N. et al. Ultralong-range energy transfer by interatomic coulombic decay in an extreme quantum system. Nat. Phys. 6, 508–511 (2010).
Gokhberg, K., Kolorenč, P., Kuleff, A. I. & Cederbaum, L. S. Site-and energy-selective slow-electron production through intermolecular coulombic decay. Nature 505, 661–663 (2014).
Havermeier, T. et al. Interatomic coulombic decay following photoionization of the helium dimer: observation of vibrational structure. Phys. Rev. Lett. 104, 133401 (2010).
Cabrera-Trujillo, R., Vendrell, O. & Cederbaum, L. S. Interatomic coulombic decay of a Li dimer in a coupled electron and nuclear dynamics approach. Phys. Rev. A 102, 032820 (2020).
LaForge, A. et al. Highly efficient double ionization of mixed alkali dimers by intermolecular coulombic decay. Nat. Phys. 15, 247–250 (2019).
Kumagai, Y. et al. Following the birth of a nanoplasma produced by an ultrashort hard-X-ray laser in xenon clusters. Phys. Rev. X 8, 031034 (2018).
Sandford, S. A., Nuevo, M., Bera, P. P. & Lee, T. J. Prebiotic astrochemistry and the formation of molecules of astrobiological interest in interstellar clouds and protostellar disks. Chem. Rev. 120, 4616–4659 (2020).
Feng, J.-Y. et al. IR-VUV spectroscopy of pyridine dimers, trimers and pyridine–ammonia complexes in a supersonic jet. Phys. Chem. Chem. Phys. 22, 21520–21534 (2020).
Ribeiro, F. A. et al. Fragment and cluster ions from gaseous and condensed pyridine produced under electron impact. Phys. Chem. Chem. Phys. 20, 25762–25771 (2018).
Scarborough, T. D., Foote, D. B. & Uiterwaal, C. J. G. J. Ultrafast resonance-enhanced multiphoton ionization in the azabenzenes: pyridine, pyridazine, pyrimidine, and pyrazine. J. Chem. Phys. 136, 054309 (2012).
Cooper, J. & Zare, R. N. Angular distribution of photoelectrons. J. Chem. Phys. 48, 942–943 (1968).
Aravind, G., Bhargava Ram, N., Gupta, A. K. & Krishnakumar, E. Probing the influence of channel coupling on the photoelectron angular distribution for the photodetachment from Cu−. Phys. Rev. A 79, 043411 (2009).
Barckholtz, C., Barckholtz, T. A. & Hadad, C. M. C–H and N–H bond dissociation energies of small aromatic hydrocarbons. J. Am. Chem. Soc. 121, 491–500 (1999).
Jabbari, G., Gokhberg, K. & Cederbaum, L. S. Competition between interatomic coulombic decay and autoionization of doubly-excited atoms. Chem. Phys. Lett. 754, 137571 (2020).
Trinter, F. et al. Vibrationally resolved decay width of interatomic coulombic decay in hene. Phys. Rev. Lett. 111, 233004 (2013).
Cherchneff, I., Barker, J. R. & Tielens, A. G. G. M. Polycyclic aromatic hydrocarbon formation in carbon-rich stellar envelopes. Astrophys. J. 401, 269 (1992).
Parker, D. S. N. et al. Low temperature formation of nitrogen-substituted polycyclic aromatic hydrocarbons (PANHs)—barrierless routes to dihydro(iso)quinolines. Astrophys. J. 815, 115 (2015).
Parker, D. S. N. et al. Gas phase synthesis of (iso)quinoline and its role in the formation of nucleobases in the interstellar medium. Astrophys. J. 803, 53 (2015).
Schmidt, M. W., Hull, E. A. & Windus, T. L. Valence virtual orbitals: an unambiguous ab initio quantification of the LUMO concept. J. Phys. Chem. A 119, 10408–10427 (2015).
Barca, G. M. J. et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 152, 154102 (2020).
Acknowledgements
This work was supported by the Department of Science and Technology through project no. EMR/2016/005247 (G.A.) and the Indian Space Research Organisation through project no. ICSR/ISRO-IITM/PHY/16-17/173/GARA (G.A. and R.K.K.).
Author information
Authors and Affiliations
Contributions
S.B., G.A. and Y.S. conceived the three-centre ICD mechanism. S.B. and G.A. planned the experiment and carried out the measurements with the support of S.D., N.R.B. and R.K.K. Y.S. planned the quantum-chemistry calculations. The results were discussed among the authors. Y.S. and G.A. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Xueguang Ren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Methods, Calculations and Computational Details.
Supplementary Data
Data used to generate figures in the Supplementary Materials file.
Source data
Source Data Fig. 1
Data used to generate Figure 1.
Source Data Fig. 2
Data used to generate Figure 2.
Source Data Fig. 3
Data used to generate Figure 3.
Source Data Fig. 5
Data used to generate Figure 5.
Rights and permissions
About this article
Cite this article
Barik, S., Dutta, S., Behera, N.R. et al. Ambient-light-induced intermolecular Coulombic decay in unbound pyridine monomers. Nat. Chem. 14, 1098–1102 (2022). https://doi.org/10.1038/s41557-022-01002-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01002-2