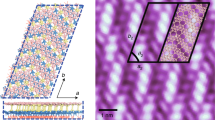
Abstract
Borophene, a crystalline monolayer boron sheet, has been predicted to adopt a variety of structures—owing to its high polymorphism—that may possess physical properties that could serve in flexible electronics, energy storage and catalysis. Several borophene polymorphs have been synthesized on noble metal surfaces but for device fabrication larger single-crystal domains are typically needed with, ideally, weak borophene–substrate interactions. Here we report the synthesis of borophene on a square-lattice Cu(100) surface and show that incommensurate coordination reduces the borophene–substrate interactions and also leads to a borophene polymorph different from those previous reported. Micrometre-scale single-crystal domains formed as isolated faceted islands or merged together to achieve full monolayer coverage. The crystal structure of this phase has ten boron atoms and two hexagonal vacancies in its unit cell. First-principles calculations indicate that charge transfer, rather than covalent bonding, binds this two-dimensional boron to the Cu(100) surface. The electronic band structure of this material features multiple anisotropic tilted Dirac cones.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are provided in the article and its Supplementary Information, and also available from the authors on request. Source data are provided with this paper.
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Novoselov, K. S. et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature 438, 197–200 (2005).
Feng, B. et al. Evidence of silicene in honeycomb structures of silicon on Ag(111). Nano Lett. 12, 3507–3511 (2012).
Meng, L. et al. Buckled silicene formation on Ir(111). Nano Lett. 13, 685–690 (2013).
Li, L. F. et al. Buckled germanene formation on Pt(111). Adv. Mater. 26, 4820 (2014).
Dávila, M. E. et al. Germanene: a novel two-dimensional germanium allotrope akin to graphene and silicene. New J. Phys. 16, 095002 (2014).
Zhu, F.-f et al. Epitaxial growth of two-dimensional stanene. Nat. Mater. 14, 1020 (2015).
Deng, J. et al. Epitaxial growth of ultraflat stanene with topological band inversion. Nat. Mater. 17, 1081–1086 (2018).
Tang, H. & Ismail-Beigi, S. Novel precursors for boron nanotubes: the competition of two-center and three-center bonding in boron sheets. Phys. Rev. Lett. 99, 115501 (2007).
Tang, H. & Ismail-Beigi, S. Self-doping in boron sheets from first principles: a route to structural design of metal boride nanostructures. Phys. Rev. B 80, 134113 (2009).
Penev, E. S., Bhowmick, S., Sadrzadeh, A. & Yakobson, B. I. Polymorphism of two-dimensional boron. Nano Lett. 12, 2441–2445 (2012).
Gao, M., Li, Q.-Z., Yan, X.-W. & Wang, J. Prediction of phonon-mediated superconductivity in borophene. Phys. Rev. B 95, 024505 (2017).
Penev, E. S., Kutana, A. & Yakobson, B. I. Can two-dimensional boron superconduct? Nano Lett. 16, 2522–2526 (2016).
Gupta, S., Kutana, A. & Yakobson, B. I. Dirac cones and nodal Line in borophene. J. Phys. Chem. Lett. 9, 2757–2762 (2018).
Feng, B. et al. Dirac fermions in borophene. Phys. Rev. Lett. 118, 096401 (2017).
Feng, B. et al. Discovery of 2D anisotropic dirac cones. Adv. Mater. 30, 1704025 (2018).
Zhang, Z., Yang, Y., Penev, E. S. & Yakobson, B. I. Elasticity, flexibility, and ideal strength of borophenes. Adv. Funct. Mater. 27, 1605059 (2017).
Jiang, H. R. et al. Borophene: a promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 23, 97–104 (2016).
Shukla, V. et al. Toward the realization of 2D borophene based gas sensor. J. Phys. Chem. C 121, 26869–26876 (2017).
Zhang, Z., Penev, E. S. & Yakobson, B. I. Two-dimensional materials: polyphony in B flat. Nat. Chem. 8, 525–527 (2016).
Sun, X. et al. Two-dimensional boron crystals: structural stability, tunable properties, fabrications and applications. Adv. Funct. Mater. 27, 1603300 (2017).
Liu, Y., Penev, E. S. & Yakobson, B. I. Probing the synthesis of two-dimensional boron by first-principles computations. Angew. Chem. 125, 3238–3241 (2013).
Mannix, A. J. et al. Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350, 1513–1516 (2015).
Feng, B. et al. Experimental realization of two-dimensional boron sheets. Nat. Chem. 8, 563 (2016).
Liu, X. et al. Intermixing and periodic self-assembly of borophene line defects. Nat. Mater. 17, 783–788 (2018).
Liu, X. et al. Self-assembly of electronically abrupt borophene/organic lateral heterostructures. Sci. Adv. 3, e1602356–e1602356 (2017).
Liu, X. & Hersam, M. C. Borophene-graphene heterostructures. Sci. Adv. 5, eaax6444 (2019).
Wu, R. et al. Large-area single-crystal sheets of borophene on Cu(111) surfaces. Nat. Nanotechnol. 14, 44–49 (2019).
Wu, R., Gozar, A. & Božović, I. Large-area borophene sheets on sacrificial Cu(111) films promoted by recrystallization from subsurface boron. npj Quantum Mater. 4, 40 (2019).
Gross, L. et al. High-resolution molecular orbital imaging using a p-wave STM tip. Phys. Rev. Lett. 107, 086101 (2011).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Zhao, F. et al. Graphene-nanodiamond heterostructures and their application to high current devices. Sci. Rep. 5, 13771 (2015).
Fei, F. et al. Band structure perfection and superconductivity in type-II Dirac semimetal Ir1−xPtxTe2. Adv. Mater. 30, 1801556 (2018).
Soluyanov, A. A. et al. Type-II Weyl semimetals. Nature 527, 495–498 (2015).
Volovik, G. E. Black hole and hawking radiation by type-II Weyl fermions. JETP Lett. 104, 645–648 (2016).
Huang, H., Jin, K.-H. & Liu, F. Black-hole horizon in the Dirac semimetal Zn2In2S5. Phys. Rev. B 98, 121110 (2018).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Zahl, P. & Wagner, T. GXSM—smart and customizable SPM control. Wiley Analytical Science Magazine (26 January 2015).
Zahl, P., Wagner, T., Möller, R. & Klust, A. Open source scanning probe microscopy control software package GXSM. J. Vac.Sci. Technol. B 28, C4E39 (2010).
Zahl, P., Bierkandt, M., Schröder, S. & Klust, A. The flexible and modern open source scanning probe microscopy software package GXSM. Rev. Sci. Instr. 74, 1222 (2003).
Chen., C. J. Effects of m ≠ 0 tip states in scanning tunneling microscopy: the explanations of corrugation reversal. Phys. Rev. Lett. 69, 1656–1659 (1992).
Guo, Q. et al. Bias dependence of apparent layer thickness and Moiré pattern on NaCl/Cu(001). Surf. Sci. 604, 1820–1824 (2010).
Faulkner, A. & Chavez, C. Adobe Photoshop CC: Classroom in a Book (2018 release) (Adobe Press, 2018).
Tersoff, J. & Hamann, D. R. Theory and application for the scanning tunneling microscope. Phys. Rev. Lett. 50, 1998–2001 (1983).
Chen, C. J. Tunneling matrix elements in three-dimensional space: the derivative rule and the sum rule. Phys. Rev. B 42, 8841–8857 (1990).
Acknowledgements
This research was supported by the US Department of Energy, Basic Energy Sciences, Materials Sciences and Engineering Division. R.W. and A.G. were supported by the Gordon and Betty Moore Foundation’s EPiQS Initiative through grant no. GBMF9074. S.E. acknowledges support from the NSF Graduate Research Fellowship (grant no. DGE1752134). I.K.D. acknowledges the financial support of the BNL Gertrude and Maurice Goldhaber Distinguished Fellowship. This research used resources of the Centre for Functional Nanomaterials and National Synchrotron Light Source II, which are US DOE Office of Science Facilities at Brookhaven National Laboratory, under contract no. DE-SC0012704. The computations used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant no. ACI-1548562, via computer time on the Comet supercomputer as enabled by XSEDE allocation MCA08X007.
Author information
Authors and Affiliations
Contributions
R.W. and I.B. designed the research strategy. R.W. performed the LEEM/LEED measurements and performed the data analysis with help from A.G. R.W. acquired and analysed the XPS data with help from J.T.S. I.K.D. and P.Z. carried out the low-temperature STM measurements, and R.W. performed the data analysis. S.E., R.W, A.G. and S.I-B. developed the atomic model. S.E. performed ab initio calculations with help from S.I-B. I.B. supervised the project. R.W. and I.B. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Snapshots of dissolution and resurfacing of borophene on Cu(100) as a function of temperature.
Panels a to l show bright-field LEEM images (FOV = 10 μm) of a B/Cu(100) sample as the substrate temperature is raised from (a) T = 758 K to (b) T = 813 K, (c) T = 871 K, (d) T = 881 K, (e) T = 893 K, and (f) T = 903 K, followed by cooling down to (g) T = 858 K, (h) T = 856 K, (i) T = 839 K, (j) T = 813 K, and (k) T = 788 K. (l) LEED pattern taken on the resurfaced boron sheet. Borophene appears dark grey and the copper substrate light grey. As the substrate temperature exceeds T = 871 K (the panel c), borophene dissolves and diffuses into the bulk of the substrate. Boron atoms migrate back to the surface upon cooling, with the first borophene islands appearing at T ≈ 858 K (see the panel h). This process can be repeated a few times before the fraction of re-emerged crystalline borophene layer becomes too small to observe, because concomitantly with the sub-surface dissolution some boron also evaporates from the surface. The LEED pattern taken on the resurfaced boron film is identical to that taken on borophene directly grown on Cu(100), confirming that the same borophene phase is formed in both cases. A real-time movie of this process is presented in “Supplementary Video 2”.
Extended Data Fig. 2 A stability test of borophene under exposure to oxygen.
a, A bright-field LEEM image of borophene islands before exposure to oxygen. Borophene appears dark grey and the copper substrate light grey. Step edges on copper substrate are highlighted by the white arrows. b, A bright-field LEEM image of the same borophene islands after exposure to the partial pressure of 1×10-6 Torr of oxygen for 1 hour. The size and shape of borophene islands remain the same, while the step edges of copper substrate become blurry. This indicates that borophene islands are stable in a mild oxygen environment and are more robust against oxidation than the Cu(100) step edges.
Extended Data Fig. 3 STM morphology of borophene island edges on Cu(100) surface.
a, The junction area of two borophene islands (yellow dashed line) as well as their edges on Cu(100) surface (white dashed line). The yellow arrow shows the Cu(110). The angles (counterclockwise) from the Cu(011) direction to the directions of the faceted island edges are 105 ± 2° and 163 ± 2° respectively. The unit cells of the super-modulation in two borophene domains are highlighted by green and red rhomboids, respectively. The borophene island edges are parallel to the long side of the corresponding large-scale super-modulations. b, The STM morphology of borophene island edge (white dashed line) on Cu(100) surface. The unit cell of the super-modulation is highlighted by a green rhomboid, and its long side is parallel to the island edge. The scanning parameters are Vb = + 500 mV, It = 200 pA for (a); Vb = + 100 mV, It = 20 pA for (b).
Extended Data Fig. 4 Dirac cones in the electron band structure of the free standing β13 borophene.
a, The 2D distribution of energy differences between neighbouring electronic Band18 and Band17. b, The same as in panel a, but for Band17 - Band16. The black solid lines reveal the high-symmetry directions for the band structure in Fig. 5a (Г-X-M-Г-Y-M). The four Dirac cones are highlighted by the red circles. The velocities of electrons on the upper cone around the Dirac points along direction 1–11 are (1): vF = 6.3 × 105 m/s, (2): 8.1 × 105 m/s, (3): 6.8 × 105 m/s, (4): 7.4 × 105 m/s, (5): 8.2 × 105 m/s, (6): -2.5 × 105 m/s, (7): the dispersion in this direction is not really linear in the immediate vicinity of the crossing; farther away from the crossing the velocity is roughly 1.7 × 105 m/s, (8): 1.3 × 106 m/s, (9): 4.4 × 105 m/s, (10): 1.2 × 106 m/s, and (11): 1.6 × 105 m/s, respectively. Side-view orientations are indicated in the panel b by the blue arrows. c, The side view of the Dirac cone ΓX from the 37.5° direction. d, The same as in the panel c, but from the 0° direction. This cone is roughly isotropic; the electron velocities in different orientations are within the range 6.5 × 105 m/s < vF < 8.1 × 105 m/s. e, The side view of the Dirac cone XM from the 0° direction. f, The same as in the panel e, from the 90° direction. g, The side view of the Dirac cone kk from the 0° direction. h, The same as in the panel g, from the 37.5° direction. i, The side view of the Dirac cone ΓY from the 37.5° direction. j, The same as in the panel i, from the 90° direction.
Extended Data Fig. 5 Real-space distribution of the electron DOS related to different Dirac points.
a, The DOS distribution in the electron Band17 related to the Dirac Cone ΓX. b, The same as in panel a, for the Band18. Dirac fermions originate from the overlap area; they mainly come from the boron honeycomb lattice (highlighted by the red dots). Pink rectangles highlight the unit cell of the new β13 borophene phase. c, The DOS distribution in Band16 related to the Dirac cone XM. d, The same as in panel c, for the Band17. The overlapped areas form an armchair structure (highlighted by the red dots). This cone largely derives from armchair-shaped chains of boron atoms, running along the short axis of the unit cell, e, The DOS distribution in Band16 related to the Dirac cone kk. f, The same as in panel e, for the Band17. The overlapped areas contain three boron atoms (highlighted by the red dots) around each vacancy. g, The DOS distribution in Band17 related to the Dirac cone ΓY. h, The same as in panel g, for the Band18. The overlapped area (highlighted by red dots) shows a zig-zag pattern. The boron atom that forms six bonds with the neighbour atoms contributes to this Dirac cone.
Extended Data Fig. 6 The atomic model for the super-modulation of borophene on Cu(100).
a, The top view of the model. There are 201 boron atoms in this big unit cell. b, A side view of the same model. The Z modulation of borophene sheet is less than 0.31 Å. The average distance between the boron layer and the top copper layer is 1.92 Å, which is around 10% longer than the copper interlayer distance ~1.77 Å. c, The statistical distribution of boron-boron bonds length. The majority of B-B bond lengths are between 1.7 and 1.8 Å, and the average bond length is 1.74 Å. The fact that the B-Cu distance is longer than the B-B distance in the borophene-on-Cu(100) system indicates that boron prefers to form a coherent borophene sheet rather than to form discrete B-Cu bonds, and that the coherent borophene sheet as a whole receives electron doping from the copper substrate. We also notice that in some metal-B complexes in fluid solution system, boron could form some very long “bonds” with the center metal atoms, for example, the one-electron σ B-Cu bonding (~2.289 Å) and two-electron σ B-Cu bonding (~2.189 Å) (supplementary Reference 1). However, such kinds of “coordinate covalent bonds” between central metal atoms and the ligands in metal complexes system are weak and flexible, hence rather different from the robust, rigid covalent chemical bonds within the borophene sheet (~1.74 Å). Our DFT calculations indicate a larger separation (~1.92 Å) and the absence of covalent bonding between copper and borophene, just some charge transfer across the boron-copper interface.
Extended Data Fig. 7 Electronic band structure of modulated β13 borophene sheet, after taking into account the stripe modulations.
a, The electron band structure of the modulated β13 borophene, computed by unfolding the spectral function of the modulated supercell onto the Brillouin zone of the 10-atom small unit cell (supplementary refers #2). The band structure follows the same high-symmetry directions as that in Fig. 5a. b, Superposition of the free-standing (red lines) and modulated β13 borophene electronic band structures along some high-symmetry directions, as indicated in the inset (the red dots correspond to the reciprocal lattice). c, d, The crosscut of the Dirac cone ГX along different directions. This Dirac cone is preserved under the striped modulation, and it just shifts a little bit in the energy-momentum space. e, f, The crosscut around the original Dirac cone XM along different directions. This Dirac crossing evolves into a Dirac nodal line due to the vertical replicas of the two bands at different energies. Moreover, some flat band features emerge in the vicinity in the E-k space, as highlighted by the red and yellow dashed lines. g, h, The crosscut of the Dirac cone kk along different directions. This Dirac cone is preserved under the striped modulation, and it also shifts a little in the E-k space. i, j, The crosscut around the Dirac cone ГY along different directions. This Dirac cone is preserved under the striped modulation, and it shifts a little bit in the E-k space. In the vicinity of this crossing, we see additional cross points by the band replicas, which makes this quantum material potentially interesting. Overall, by breaking the translation symmetry of the small unit cell, the striped features indeed have a substantial influence on the band structure of the β13 boron sheet. Specifically, band shifts/replicas in the E-k space produce gap openings, flat bands, abundant degeneracy points and lines, and make this system a promising candidate to explore novel emergent physics.
Supplementary information
Supplementary Information
Supplementary Figures 1–3, Supplementary Table 1, Supplementary Discussion and all source data.
Supplementary Video 1
Coloured version of video 1: Borophene growth on Cu(100). A real-time LEEM movie showing growth of a full monolayer of borophene. The field of view is 3 µm. The Cu(100) substrate was kept at ~750 K. This video plays at ~100 times the actual speed of the borophene growth process.
Supplementary Video 2
Coloured version: Borophene dissolution and resurfacing. A LEEM movie showing the dissolution of borophene into the bulk of Cu as the temperature is ramped up to ~870 K, followed by resurfacing of crystalline borophene when the sample is cooled down below ~750 K.
Supplementary Video 3
Source of video 1 (uncoloured version): Borophene growth on Cu(100). A real-time LEEM movie showing growth of a full monolayer of borophene. The field of view is 3 µm. The Cu(100) substrate was kept at ~750 K. This video plays at ~100 times the actual speed of the borophene growth process.
Supplementary Video 4
Source of video 2 (uncoloured version): Borophene dissolution and resurfacing. A LEEM movie showing the dissolution of borophene into the bulk of Cu as the temperature is ramped up to ~870 K, followed by resurfacing of crystalline borophene when the sample is cooled down below ~750 K.
Source data
Source Data Fig. 1
Raw (unprocessed) LEEM images.
Source Data Fig. 2
Raw (unprocessed) LEEM and LEED images.
Source Data Fig. 3
Raw (unprocessed) STM images.
Source Data Fig. 4
Raw (unprocessed) STM images.
Source Data Extended Data Fig. 1
Raw (unprocessed) LEEM and LEED images.
Source Data Extended Data Fig. 2
Raw (unprocessed) LEEM images.
Source Data Extended Data Fig. 3
Raw (unprocessed) STM images.
Source Data Extended Data Fig. 6
Atomic model source data files (in.txt format).
Rights and permissions
About this article
Cite this article
Wu, R., Eltinge, S., Drozdov, I.K. et al. Micrometre-scale single-crystalline borophene on a square-lattice Cu(100) surface. Nat. Chem. 14, 377–383 (2022). https://doi.org/10.1038/s41557-021-00879-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00879-9
This article is cited by
-
Epitaxial growth of borophene on graphene surface towards efficient and broadband photodetector
Nano Research (2024)
-
Experimental evidence of surface copper boride
Nano Research (2023)