Abstract
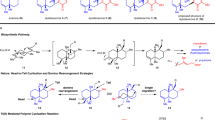
Natural product total synthesis inspires the development of synthesis strategies to access important classes of molecules. In the 1960s, Corey and coworkers demonstrated a visionary preparation of the terpenoid longifolene, using ‘strategic bond analysis’ to craft a synthesis route. This approach proposes that efficient synthesis routes to bridged, polycyclic structures should be formulated to introduce the bulk of the target’s topological complexity at a late stage. Subsequently, similar strategies have proved general for the syntheses of a wide variety of bridged, polycyclic molecules. Here, we demonstrate that an orthogonal strategy where topological complexity is introduced at the outset leads to the short synthesis of the longifolene-related terpenoid longiborneol. To implement this strategy, we access a bicyclo[2.2.1] starting material through scaffold remodelling of readily available (S)-carvone. We also employ a variety of late-stage C–H functionalization tactics in divergent syntheses of many longiborneol congeners. Our strategy may prove effective for the preparation of other topologically complex natural products that contain the bicyclo[2.2.1] framework.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2060682 (11) and 2060683 (10). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The experimental procedures and characterization of all compounds are provided in the Supplementary Information.
References
Brill, Z. G., Condakes, M. L., Ting, C. P. & Maimone, T. J. Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem. Rev. 117, 11753–11795 (2017).
Corey, E. J., Ohno, M., Vatakencherry, P. A. & Mitra, R. B. Total synthesis of d,l-longifolene. J. Am. Chem. Soc. 83, 1251–1253 (1961).
Corey, E. J., Ohno, Masaji, Mitra, R. B. & Vatakencherry, P. A. Total synthesis of longifolene. J. Am. Chem. Soc. 86, 478–485 (1964).
Corey, E. J., Howe, W. J., Orf, H. W., Pensak, D. A. & Petersson, G. General methods of synthetic analysis. Strategic bond disconnections for bridged polycyclic structures. J. Am. Chem. Soc. 97, 6116–6124 (1975).
Boger, D. L. in Modern Organic Synthesis Lecture Notes (ed. Garbaccio, R. M.) 443–460 (TSRI Press, 1999).
Doering, N. A., Sarpong, R. & Hoffmann, R. W. A case for bond-network analysis in the synthesis of bridged polycyclic complex molecules: hetidine and hetisine diterpenoid alkaloids. Angew. Chem. Int. Ed. 59, 10722–10731 (2020).
Marth, C. J. et al. Network-analysis-guided synthesis of weisaconitine D and liljestrandinine. Nature 528, 493–498 (2015).
Haider, M., Sennari, G., Eggert, A. & Sarpong, R. Total synthesis of the cephalotaxus norditerpenoids (±)-cephanolides A–D. J. Am. Chem. Soc. 143, 2710–2715 (2021).
Matsuo, A., Nakayama, M. & Hayashi, S. Chemical proof of enantiomeric (−)-longiborneol. Chem. Lett. 2, 769–772 (1973).
Takasu, K., Mizutani, S., Noguchi, M., Makita, K. & Ihara, M. Stereocontrolled total synthesis of (±)-culmorin via the intramolecular double Michael addition. Org. Lett. 1, 391–394 (1999).
Takasu, K., Mizutani, S., Noguchi, M., Makita, K. & Ihara, M. Total synthesis of (±)-culmorin and (±)-longiborneol: an efficient construction of tricyclo[6.3.0.03,9]undecan-10-one by intramolecular double Michael addition. J. Org. Chem. 65, 4112–4119 (2000).
Kuo, D. L. & Money, T. An enantiospecific synthesis of longiborneol and longifolene. J. Chem. Soc. Chem. Commun. 0, 1691–1692 (1986).
Kuo, D. L. & Money, T. Enantiospecific synthesis of longiborneol and longifolene. Can. J. Chem. 66, 1794–1804 (1988).
Masarwa, A., Weber, M. & Sarpong, R. Selective C–C and C–H bond activation/cleavage of pinene derivatives: synthesis of enantiopure cyclohexenone scaffolds and mechanistic insights. J. Am. Chem. Soc. 137, 6327–6334 (2015).
Weber, M., Owens, K., Masarwa, A. & Sarpong, R. Construction of enantiopure taxoid and natural product-like scaffolds using a C–C bond cleavage/arylation reaction. Org. Lett. 17, 5432–5435 (2015).
Kuroda, Y. et al. Isolation, synthesis and bioactivity studies of phomactin terpenoids. Nat. Chem. 10, 938–945 (2018).
Kerschgens, I., Rovira, A. R. & Sarpong, R. Total synthesis of (−)-xishacorene B from (R)-carvone using a C–C activation strategy. J. Am. Chem. Soc. 140, 9810–9813 (2018).
Bahadoor, A. et al. Hydroxylation of longiborneol by a Clm2-encoded CYP450 monooxygenase to produce culmorin in Fusarium graminearum. J. Nat. Prod. 79, 81–88 (2016).
Ashley, J. N., Hobbs, B. C. & Raistrick, H. Studies in the biochemistry of micro-organisms: the crystalline colouring matters of Fusarium culmorum (W. G. Smith) Sacc. and related forms. Biochem. J. 31, 385–397 (1937).
Alam, M., Jones, E. B. G., Hossain, M. B. & van der Helm, D. Isolation and structure of isoculmorin from the marine fungus Kallichroma tethys. J. Nat. Prod. 59, 454–456 (1996).
Kasitu, G. C. et al. Isolation and characterization of culmorin derivatives produced by Fusariumculmorum CMI 14764. Can. J. Chem. 70, 1308–1316 (1992).
Chen, K. & Baran, P. S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature 459, 824–828 (2009).
White, M. C. & Zhao, J. Aliphatic C–H oxidations for late-stage functionalization. J. Am. Chem. Soc. 140, 13988–14009 (2018).
Ma, X., Kucera, R., Goethe, O. F., Murphy, S. K. & Herzon, S. B. Directed C–H bond oxidation of (+)-pleuromutilin. J. Org. Chem. 83, 6843–6892 (2018).
Qiu, Y. & Gao, S. Trends in applying C–H oxidation to the total synthesis of natural products. Nat. Prod. Rep. 33, 562–581 (2016).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Welch, S. C. & Walters, R. L. Total syntheses of (+)-longicamphor and (+)-longiborneol. Synth. Commun. 3, 419–423 (1973).
Welch, S. C. & Walters, R. L. Stereoselective total syntheses of (±)-longicyclene, (±)-longicamphor, and (±)-longiborneol. J. Org. Chem. 39, 2665–2673 (1974).
Wittig, G. & Schöllkopf, U. Über triphenyl-phosphin-methylene als olefinbildende reagenzien (I. Mitteil. Chem. Ber. 87,1318–1330 (1954).
Bermejo, F. A. et al. Ti(III)-promoted cyclizations. Application to the synthesis of (E)-endo-bergamoten-12-oic acids. Moth oviposition stimulants isolated from Lycopersicon hirsutum. Tetrahedron 62, 8933–8942 (2006).
Song, Z.-L., Fan, C.-A. & Tu, Y.-Q. Semipinacol rearrangement in natural product synthesis. Chem. Rev. 111, 7523–7556 (2011).
Ikan, R., Markus, A. & Bergmann, E. D. The synthesis of 22-trans-cholesta-5,22,25-trien-3β OL. Isr. J. Chem. 9, 259–261 (1971).
Huang, Q., Suravarapu, S. R. & Renaud, P. A Giese reaction for electron-rich alkenes. Chem. Sci. https://doi.org/10.1039/D0SC06341J (2021).
Song, H.-J., Lim, C. J., Lee, S. & Kim, S. Tin-free radical alkylation of ketones via N-silyloxy enamines. Chem. Commun. 2893–2895 (2006).
Lee, J. Y., Lim, K.-C., Meng, X. & Kim, S. Radical alkylations of alkyl halides and unactivated C-H bonds using vinyl triflates. Synlett 2010, 1647–1650 (2010).
Roberts, B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Crossley, S. W. M., Barabé, F. & Shenvi, R. A. Simple, chemoselective, catalytic olefin isomerization. J. Am. Chem. Soc. 136, 16788–16791 (2014).
Fu, M.-C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
Lo, J. C., Gui, J., Yabe, Y., Pan, C.-M. & Baran, P. S. Functionalized olefin cross-coupling to construct carbon–carbon bonds. Nature 516, 343–348 (2014).
Leggans, E. K., Barker, T. J., Duncan, K. K. & Boger, D. L. Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20′-vinblastine analogues. Org. Lett. 14, 1428–1431 (2012).
Lo, J. C., Yabe, Y. & Baran, P. S. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 136, 1304–1307 (2014).
Isayama, S. & Mukaiyama, T. A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 1071–1074 (1989).
Concepción, J. I., Francisco, C. G., Hernández, R., Salazar, J. A. & Suárez, E. Intramolecular hydrogen abstraction. Iodosobenzene diacetate, an efficient and convenient reagent for alkoxy radical generation. Tetrahedron Lett. 25, 1953–1956 (1984).
Nakazaki, M. & Naemura, K. Photolyses of isobornyl and bornyl nitrites. Bull. Chem. Soc. Jpn 37, 532–535 (1964).
Renata, H., Zhou, Q. & Baran, P. S. Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide. Science 339, 59–63 (2013).
Berger, M., Knittl-Frank, C., Bauer, S., Winter, G. & Maulide, N. Application of relay C−H oxidation logic to polyhydroxylated oleanane triterpenoids. Chem 6, 1183–1189 (2020).
Desai, L. V., Hull, K. L. & Sanford, M. S. Palladium-catalyzed oxygenation of unactivated sp3 C−H bonds. J. Am. Chem. Soc. 126, 9542–9543 (2004).
Neufeldt, S. R. & Sanford, M. S. O-acetyl oximes as transformable directing groups for Pd-catalyzed C−H bond functionalization. Org. Lett. 12, 532–535 (2010).
Caglioti, L. & Magi, M. The reaction of tosylhydrazones with lithium aluminium hydride. Tetrahedron 19, 1127–1131 (1963).
Simmons, E. M. & Hartwig, J. F. Catalytic functionalization of unactivated primary C–H bonds directed by an alcohol. Nature 483, 70–73 (2012).
Li, B., Driess, M. & Hartwig, J. F. Iridium-catalyzed regioselective silylation of secondary alkyl C–H bonds for the synthesis of 1,3-diols. J. Am. Chem. Soc. 136, 6586–6589 (2014).
Tamao, K., Ishida, N. & Kumada, M. (Diisopropoxymethylsilyl)methyl Grignard reagent: a new, practically useful nucleophilic hydroxymethylating agent. J. Org. Chem. 48, 2120–2122 (1983).
Fleming, I., Henning, R. & Plaut, H. The phenyldimethylsilyl group as a masked form of the hydroxy group. J. Chem. Soc. Chem. Commun. 29–31 (1984).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Curci, R., D’Accolti, L. & Fusco, C. A novel approach to the efficient oxygenation of hydrocarbons under mild conditions. Superior oxo transfer selectivity using dioxiranes. Acc. Chem. Res. 39, 1–9 (2006).
Acknowledgements
We are grateful to the National Institutes of Health (NIGMS MIRA R35 GM130345) for grant support as well as the Molecular Scaffold Design Collective (agreement no. HR00111890024) of the Defense Advanced Research Projects Agency for partial support of this work. R.F.L. is grateful for fellowship support from the National Science Foundation Graduate Research Fellowships Program (DGE 1752814). G.S. thanks the Uehara Memorial Foundation for a postdoctoral fellowship. We thank H. Celik and University of California, Berkeley’s NMR facility in the College of Chemistry (CoC-NMR) for spectroscopic assistance. Instruments in the CoC-NMR are supported in part by National Institutes of Health S10OD024998. We thank N. Settineri (University of California, Berkeley) for single-crystal X-ray diffraction studies.
Author information
Authors and Affiliations
Contributions
The overall design for this project was conceptualized by G.S. with input from R.F.L. and R.S.; R.F.L. and G.S. conducted the chemical reactions. G.S. and R.F.L. developed the synthesis of 15 and 3, and G.S. optimized the route. Syntheses of 5 and 10–14 were achieved by R.F.L., who also designed the C–H functionalization sequences with input from G.S. and R.S. Rearrangement of 3 to 1 as well as preparation of 38 and 41 were discovered by G.S. The manuscript was written and edited jointly by R.F.L., G.S. and R.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Kiyosei Takasu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Tables 1–8, experimental procedures, NMR data and crystallographic data.
Supplementary Data 1
Crystallographic data for compound 10; CCDC reference 2060683.
Supplementary Data 2
Crystallographic data for compound 11; CCDC reference 2060682.
Rights and permissions
About this article
Cite this article
Lusi, R.F., Sennari, G. & Sarpong, R. Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy. Nat. Chem. 14, 450–456 (2022). https://doi.org/10.1038/s41557-021-00870-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00870-4
This article is cited by
-
Strategic application of C–H oxidation in natural product total synthesis
Nature Reviews Chemistry (2023)