Abstract
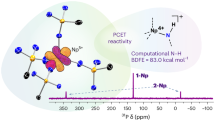
Neptunium was the first actinide element to be artificially synthesized, yet, compared with its more famous neighbours uranium and plutonium, is less conspicuously studied. Most neptunium chemistry involves the neptunyl di(oxo)-motif, and transuranic compounds with one metal–ligand multiple bond are rare, being found only in extended-structure oxide, fluoride or oxyhalide materials. These combinations stabilize the required high oxidation states, which are otherwise challenging to realize for transuranic ions. Here we report the synthesis, isolation and characterization of a stable molecular neptunium(V)–mono(oxo) triamidoamine complex. We describe a strong Np≡O triple bond with dominant 5f-orbital contributions and σu > πu energy ordering, akin to terminal uranium-nitrides and di(oxo)-actinyls, but not the uranium–mono(oxo) triple bonds or other actinide multiple bonds reported so far. This work demonstrates that molecular high-oxidation-state transuranic complexes with a single metal–ligand bond can be stabilized and studied in isolation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2055264 (2) and 2055265 (3). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are presented in the main text and the Supplementary Information tables, and are also available from the corresponding authors on reasonable request.
References
Ibers, J. Neglected neptunium. Nat. Chem. 2, 996–996 (2010).
Nugent, W. A. & Mayer, J. M. Metal-Ligand Multiple Bonds (Wiley, 1988).
Hayton, T. W. Metal-ligand multiple bonding in uranium: structure and reactivity. Dalton Trans. 39, 1145–1158 (2010).
Jones, M. B. & Gaunt, A. J. Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chem. Rev. 113, 1137–1198 (2013).
Hayton, T. W. Recent developments in actinide-ligand multiple bonding. Chem. Commun. 49, 2956–2973 (2013).
La Pierre, H. S. & Meyer, K. Activation of small molecules by molecular uranium complexes. Prog. Inorg. Chem. 58, 303–415 (2014).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Ephritikhine, M. The vitality of uranium molecular chemistry at the dawn of the XXIst century. Dalton Trans. 2006, 2501–2516 (2006).
Kovács, A., Konings, R. J. M., Gibson, J. K., Infante, I. & Gagliardi, L. Quantum chemical calculations and experimental investigations of molecular actinide oxides. Chem. Rev. 115, 1725–1759 (2015).
Brown, D., Reynolds, C. T. & Moseley, P. T. Crystal structure of bis(tetraethylammonium) oxopentachloroprotactinate(V). J. Chem. Soc. Dalton Trans. 1972, 857–859 (1972).
Le Naour, C. et al. First structural characterization of a protactinium(V) single oxo bond in aqueous media. Inorg. Chem. 44, 9542–9546 (2005).
Ma, G., Ferguson, M. J. & Cavell, R. G. Actinide metals with multiple bonds to carbon: synthesis, characterization, and reactivity of U(IV) and Th(IV) bis(iminophosphorano)methandiide pincer carbene complexes. Inorg. Chem. 50, 6500–6508 (2011).
Ren, W., Deng, X., Zi, G. & Fang, D.-C. The Th=C double bond: an experimental and computational study of thorium poly-carbene complexes. Dalton Trans. 40, 9662–9664 (2011).
Ren, W., Zi, G., Fang, D.-C. & Walter, M. D. Thorium oxo and sulfido metallocenes: synthesis, structure, reactivity, and computational studies. J. Am. Chem. Soc. 133, 13183–13196 (2011).
Bell, N., Maron, L. & Arnold, P. L. Thorium mono- and bis(imido) complexes made by reprotonation of cyclo-metalated amides. J. Am. Chem. Soc. 137, 10492–10495 (2015).
Smiles, D. E., Wu, G., Kaltsoyannis, N. & Hayton, T. W. Thorium-ligand multiple bonds via reductive deprotection of a trityl group. Chem. Sci. 6, 3891–3899 (2015).
Dau, P. D., Wilson, R. E. & Gibson, J. K. Elucidating protactinium hydrolysis: the relative stabilities of PaO2(H2O)+ and PaO(OH)2. Inorg. Chem. 54, 7474–7480 (2015).
Smiles, D. W., Wu, G., Hrobárik, P. & Hayton, T. W. Use of 77Se and 125Te NMR spectroscopy to probe covalency of the actinide-chalcogen bonding in [Th(En){N(SiMe3)2}3]− (E = Se, Te; n = 1, 2) and their oxo-uranium(VI) congeners. J. Am. Chem. Soc. 138, 814–825 (2016).
Gregson, M. et al. The inverse-trans-influence in tetravalent lanthanide and actinide bis(carbene) complexes. Nat. Commun. 8, 14137 (2017).
Smiles, D. E., Wu, G., Hrobárik, P. & Hayton, T. W. Synthesis, thermochemistry, bonding, and 13C NMR chemical shift analysis of a phosphorano-stabilized carbene of thorium. Organometallics 36, 4519–4524 (2017).
Rungthanaphatsophon, P. et al. Formation of methane versus benzene in the reactions of (C5Me5)2Th(CH3)2 with [CH3PPh3]X (X = Cl, Br, I) yielding thorium-carbene or thorium-ylide complexes. Angew. Chem. Int. Ed. 56, 12925–12929 (2017).
Morss, L. R. et al. (eds) The Chemistry of the Actinide and Transactinide Elements 3rd edn (Springer, 2006).
Arnold, P. L., Dutkiewicz, M. S. & Walter, O. Organometallic neptunium chemistry. Chem. Rev. 117, 11460–11475 (2017).
Gibson, J. K. Gas-phase transuranium organometallic chemistry: reactions of Np+, Pu+, NpO+ and PuO+ with alkenes. J. Am. Chem. Soc. 120, 2633–2640 (1998).
Gibson, J. K. Actinide gas-phase chemistry: reactions of An+ and AnO+ [An = Th, U, Np, Pu, Am] with nitriles and butylamine. Inorg. Chem. 38, 165–173 (1999).
Gibson, J. K. et al. Gas-phase reactions of hydrocarbons with An+ and AnO+ (An = Th, Pa, U, Np, Pu, Am, Cm): the active role of 5f electrons in organoprotactinium chemistry. Organometallics 26, 3947–3956 (2007).
Marçalo, J. & Gibson, J. K. Gas-phase energetics of actinide oxides: an assessment of neutral and cationic monoxides and dioxides from thorium to curium. J. Phys. Chem. A 113, 12599–12606 (2009).
Infante, I. et al. Ionization energies for the actinide mono- and dioxides series, from Th to Cm: theory versus experiment. J. Phys. Chem. A 114, 6007–6015 (2010).
Pereira, C. C. L., Marsden, C. J., Marçalo, J. & Gibson, J. K. Actinide sulfides in the gas phase: experimental and theoretical studies of the thermochemistry of AnS (An = Ac, Th, Pa, U, Np, Pu, Am and Cm). Phys. Chem. Chem. Phys. 13, 12940–12958 (2011).
Bagnall, K. W. & Laidler, J. B. Neptunium chloro-complexes. J. Chem. Soc. A 1966, 516–520 (1966).
Bagnall, K. W., Brown, D. & Easey, J. F. Neptunium(V) and (VI) oxyfluorides. J. Chem. Soc. A 1968, 2223–2227 (1968).
Forbes, T. Z., Burns, P. C., Skanthakumar, S. & Soderholm, L. Synthesis, structure and magnetism of Np2O5. J. Am. Chem. Soc. 129, 2760–2761 (2007).
Eller, P. G., Malm, J. G., Swanson, B. I. & Morss, L. R. Reactions of hexafluorides of uranium, neptunium and plutonium with nitrogen oxides and oxyfluorides. Synthesis and characterization of (NO)[NpF6] and (NO)[PuF6]. J. Alloys Comp. 269, 50–56 (1998).
Kiselev, Y. M. et al. On existence and properties of plutonium(VIII) derivatives. Radiochim. Acta 102, 227–237 (2014).
Charushnikova, I. A., Krot, N. N., Grigor’ev, M. S. & Makerenkov, V. I. New data on Np(VII) compounds with Co(NH3)63+. Crystal structure of [Co(NH3)6]3[NpO4(OH)2]3·4H2O and refinement of the structure of [Co(NH3)6][NpO4(OH)2]·2H2O. Radiochemistry 59, 124–133 (2017).
Brown, J. L. et al. A linear trans-bis(imido) neptunium(V) actinyl analog: NpV(NDipp)2(tBu2bipy)2Cl (Dipp = 2,6-iPr2C6H3). J. Am. Chem. Soc. 137, 9583–9586 (2015).
Samulski, E. T. & Karraker, D. G. Some ethoxide compounds of neptunium. J. Inorg. Nucl. Chem. 29, 993–996 (1967).
Brown, J. L. et al. Neptunium and plutonium complexes with a sterically encumbered triamidoamine (TREN) scaffold. Chem. Commun. 52, 5428–5431 (2016).
Staun, S. L. et al. Expanding the nonaqueous chemistry of neptunium: synthesis and structural characterization of [Np(NR2)3Cl], [Np(NR2)3Cl]−, and [Np{N(R)(SiMe2CH2)}2(NR2)]− (R = SiMe3). Inorg. Chem. 60, 2740–2748 (2021).
Sonnenberger, D. C. & Gaudiello, J. G. Synthesis and cyclic voltammetric study of bis(pentamethylcyclopentadienyl)neptunium dichloride. J. Less Common Metals 126, 411–414 (1986).
Sonnenberger, D. C. & Gaudiello, J. G. Cyclic voltammetric study of organoactinide compounds of uranium(IV) and neptunium(IV). Ligand effects on the M(IV)/M(III) couple. Inorg. Chem. 27, 2747–2748 (1988).
Klamm, B. E. et al. Exploring the oxidation states of neptunium with Schiff base coordination complexes. Inorg. Chem. 59, 18035–18047 (2020).
King, D. M. et al. Synthesis and structure of a terminal uranium nitride complex. Science 337, 717–720 (2012).
King, D. M. et al. Isolation and characterization of a uranium(VI)–nitride triple bond. Nat. Chem. 5, 482–488 (2013).
King, D. M. et al. Single-molecule magnetism in a single-ion triamidoamine uranium(V) terminal mono-oxo complex. Angew. Chem. Int. Ed. 52, 4921–4924 (2013).
Fortier, S., Brown, J. L., Kaltsoyannis, N., Wu, G. & Hayton, T. W. Synthesis, molecular and electronic structure of UV(O)[N(SiMe3)2]3. Inorg. Chem. 51, 1625–1633 (2012).
Pyykkö, P. Additive covalent radii for single-, double- and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J. Phys. Chem. A 119, 2326–2337 (2015).
Wang, S. et al. Neptunium diverges sharply from uranium and plutonium in crystalline borate matrixes: insights into the complex behavior of the early actinides relevant to nuclear waste storage. Angew. Chem. Int. Ed. 49, 1263–1266 (2010).
Tatsumi, K. & Hoffmann, R. Bent cis d0 MoO22+ vs. linear trans d0f0 UO22+: a significant role for nonvalence 6p orbitals in uranyl. Inorg. Chem. 19, 2656–2658 (1980).
Denning, R. G. Electronic structure and bonding in actinyl ions. Struct. Bonding 79, 215–276 (1992).
Denning, R. G. Electronic structure and bonding in actinyl ions and their analogs. J. Phys. Chem. A 111, 4125–4143 (2007).
La Pierre, H. S. & Meyer, K. Uranium-ligand multiple bonding in uranyl analogues, [L=U=L]n+, and the inverse trans influence. Inorg. Chem. 52, 529–539 (2013).
Lewis, A. J., Carroll, P. J. & Schelter, E. J. Stable uranium(VI) methyl and acetylide complexes and the elucidation of an inverse trans influence ligand series. J. Am. Chem. Soc. 135, 13185–13192 (2013).
Zi, G. et al. Preparation and reactions of base-free bis(1,2,4-tri-tert-butylcyclopentadienyl)uranium oxide, Cp'2UO. Organometallics 24, 4251–4264 (2005).
Vallet, V., Wahlgren, U. & Grenthe, I. Probing the nature of chemical bonding in uranyl(VI) complexes with quantum chemical methods. J. Phys. Chem. A 116, 12373–12380 (2012).
Castro-Rodríguez, I. & Meyer, K. Small molecule activation at uranium coordination complexes: control of reactivity via molecular architecture. Chem. Commun. 2006, 1353–1368 (2006).
Kindra, D. R. & Evans, W. J. Magnetic susceptibility of uranium complexes. Chem. Rev. 114, 8865–8882 (2014).
Seed, J. A. et al. Anomalous magnetism of uranium(IV)-oxo and -imido complexes reveals unusual doubly degenerate electronic ground states. Chem 7, 1666–1680 (2021).
King, D. M. et al. Synthesis and characterization of an f-block terminal parent imido [U=NH] complex: a masked uranium(IV) nitride. J. Am. Chem. Soc. 136, 5619–5622 (2014).
Coutinho, J. T. et al. Spectroscopic determination of the electronic structure of a uranium single-ion magnet. Chem. Eur. J. 25, 1758–1766 (2019).
Karraker, D. G. Magnetic susceptibilities of the tetravalent plutonium ion in octahedral compounds. Inorg. Chem. 10, 1564–1566 (1971).
La Pierre, H. S., Scheurer, A., Heinemann, F. W., Hieringer, W. & Meyer, K. Synthesis and characterization of a uranium(II) monoarene complex supported by δ backbonding. Angew. Chem. Int. Ed. 53, 7158–7162 (2014).
Windorff, C. J. et al. Expanding the chemistry of molecular U2+ complexes: synthesis, characterization, and reactivity of the {[C5H3(SiMe3)2]3U}− anion. Chem. Eur. J. 22, 772–782 (2016).
Billow, B. S. et al. Synthesis and characterization of a neutral U(II) arene sandwich complex. J. Am. Chem. Soc. 140, 17369–17373 (2018).
Apostolidis, C. et al. Tris-{hydridotris(1-pyrazolyl)borato}actinide complexes: synthesis, spectroscopy, crystal structure, bonding properties and magnetic behaviour. Chem. Eur. J. 26, 11293–11306 (2020).
Dutkiewicz, M. S. et al. Organometallic neptunium(III) complexes. Nat. Chem. 8, 797–802 (2016).
Aquilante, F. et al. MOLCAS 8: new capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 37, 506–541 (2016).
Te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Du, J. et al. Thorium-nitrogen multiple bonds provide evidence for pushing-from-below for early actinides. Nat. Commun. 10, 4203 (2019).
Kaltsoyannis, N. Computational study of analogues of the uranyl ion containing the −N=U=N− unit: density functional theory calculations on UO22+, UON+, UN2, UO(NPH3)24+, [UCl4{NPR3}2] (R = H, Me), and [UOCl4{NP(C6H5)3}]−. Inorg. Chem. 39, 6009–6017 (2000).
Lu, E. et al. Emergence of the structure-directing role of f-orbital overlap-driven covalency. Nat. Commun. 10, 634 (2019).
Lewis, A. J., Carroll, P. J. & Schelter, E. J. Reductive cleavage of nitrite to form terminal uranium mono-oxo complexes. J. Am. Chem. Soc. 135, 511–518 (2013).
Bader, R. F. W., Slee, T. S., Cremer, D. & Kraka, E. Description of conjugation and hyperconjugation in terms of electron distributions. J. Am. Chem. Soc. 105, 5061–5068 (1983).
Strittmatter, R. J. & Bursten, B. E. Bonding in tris(η5-cyclopentadienyl) actinide complexes. 5. A comparison of the bonding in Np, Pu, and transplutonium compounds with that in lanthanide compounds and a transition-metal analogue. J. Am. Chem. Soc. 113, 552–559 (1991).
Pepper, M. & Bursten, B. E. The electronic structure of actinide-containing molecules: a challenge to applied quantum chemistry. Chem. Rev. 91, 719–741 (1991).
Wu, Q.-Y., Wang, C.-Z., Lan, J.-H., Chai, Z.-F. & Shi, W.-Q. Electronic structures and bonding of the actinide halides An(TrenTIPS)X (An = Th–Pu; X = F–I): a theoretical perspective. Dalton Trans. 49, 15895–15902 (2020).
Tassell, M. J. & Kaltsoyannis, N. Covalency in AnCp4 (An = Th–Cm): a comparison of molecular orbital, natural population and atoms-in-molecules analyses. Dalton Trans. 39, 6719–6725 (2010).
Walensky, J. R., Martin, R. L., Ziller, J. W. & Evans, W. J. Importance of energy level matching for bonding in Th3+-Am3+ actinide metallocene amidinates, (C5Me5)2[iPrNC(Me)NiPr]An. Inorg. Chem. 49, 10007–10012 (2010).
Kirker, I. & Kaltsoyannis, N. Does covalency really increase across the 5f series? A comparison of molecular orbital, natural population, spin and electron density analyses of AnCp3 (An = Th–Cm; Cp = C5H5). Dalton Trans. 40, 124–131 (2011).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Kaltsoyannis, N. Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg. Chem. 52, 3407–3413 (2013).
Bacha, R. U. S., Bi, Y.-T., Xuan, L.-C. & Pan, Q.-J. Inverse trans influence in low-valence actinide-group 10 metal complexes of phosphinoaryl oxides: a theoretical study via tuning metals and donor ligands. Inorg. Chem. 58, 10028–10037 (2019).
Hu, S.-X., Zhang, P., Lu, E. & Zhang, P. Decisive role of 5f-orbital covalence in the structure and stability of pentavalent transuranic oxo [M6O8] clusters. Inorg. Chem. 59, 18068–18077 (2020).
Behrle, A. C., Myers, A. J., Kerridge, A. & Walensky, J. R. Coordination chemistry and QTAIM analysis of homoleptic dithiocarbamate complexes, M(S2CNiPr2)4 (M = Ti, Zr, Hf, Th, U, Np). Inorg. Chem. 57, 10518–10524 (2018).
Kloditz, R. et al. Series of tetravalent actinide amidinates: structure determination and bonding analysis. Inorg. Chem. 59, 15670–15680 (2020).
Kloditz, R. et al. Comprehensive bonding analysis of tetravalent f-element complexes of the type [M(salen)2]. Inorg. Chem. 60, 2514–2525 (2021).
Jin, G. B., Skanthakumar, S., Haire, R. G., Soderholm, L. & Ibers, J. A. Neptunium thiophosphate chemistry: intermediate behavior between uranium and plutonium. Inorg. Chem. 50, 9688–9695 (2011).
Bruker APEX2, SAINT-Plus, SADABS (Bruker AXS Inc., 2007); https://www.bruker.com/en/products-and-solutions/diffractometers-and-scattering-systems/single-crystal-x-ray-diffractometers/sc-xrd-software.html
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. C 71, 3–8 (2015).
Soltek, R. & Huttner, G. Winray-32 (Univ. Heidelberg, 1998).
Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 45, 849–854 (2012).
Persistence of Vision (TM) Raytracer (Persistence of Vision Pty Ltd); https://www.povray.org/
Speldrich, M., Leusen, Van & Kögerler, J. P. CONDON 3.0: an updated software package for magnetochemical analysis—all the way to polynuclear actinide complexes. J. Comput. Chem. 39, 2133–2145 (2018).
Popa, K. et al. A low-temperature synthesis method for AnO2 nanocrystals (An = Th, U, Np and Pu) and associate solid solutions. CrystEngComm 20, 4614–4622 (2018).
Laubereau, P. Präparative und radiochemische Synthesen von Cyclopentadienylkomplexen der Actiniden und des Promethiums, sowie Untersuchungen zum Spaltprodukteinbau in Aromaten-Fängerkomplexe (Technischen Hochschule München, 1966).
Thomson, R. K., Scott, B. L., Morris, D. E. & Kiplinger, J. L. Synthesis, structure, spectroscopy and redox energetics of a series of uranium(IV) mixed-ligand metallocene complexes. C. R. Chim. 13, 790–802 (2010).
LaHalle, M. P., Krupa, J. C., Guillaumont, R. & Rizzoli, C. Optical spectroscopy of Np4+ (5f3) ion diluted in ThSiO4 and ThO2 crystalline hosts. J. Less Common Met. 122, 65–73 (1986).
Carnall, W. T. A systematic analysis of the spectra of trivalent actinide chlorides in D3h site symmetry. J. Chem. Phys. 96, 8713–8726 (1992).
Freeman, A. J. & Keller, C. (eds) Handbook on the Physics and Chemistry of the Actinides (North Holland, 1984).
Fonseca Guerra, A. C., Snijders, J. G., Te Velde, G. & Baerends, E. J. Towards an order-N DFT method. Theor. Chem. Acc. 99, 391–403 (1998).
Van Lenthe, E., Baerends, E. J. & Snijders, J. G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 99, 4597–4610 (1993).
Van Lenthe, E., Baerends, E. J. & Snijders, J. G. Relativistic total energy using regular approximations. J. Chem. Phys. 101, 9783–9792 (1994).
Van Lenthe, E., Ehlers, A. E. & Baerends, E. J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 110, 8943–8953 (1999).
Vosko, S. H., Wilk, L. & Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 58, 1200–1211 (1980).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 38, 3098–3100 (1988).
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986).
Glendening, E. D. et al. NBO 5.0 (Theoretical Chemistry Institute, 2001); http://www.chem.wisc.edu/~nbo5
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, 1990).
Bader, R. F. W. A bond path: a universal indicator of bonded interactions. J. Phys. Chem. A 102, 7314–7323 (1998).
Ortiz Alba, J. C. & Jané, C. B. Xaim (Universitat Rovira i Virgili); http://www.quimica.urv.es/XAIM
Portmann, S. & Luthi, H. P. MOLEKEL: an interactive molecular graphics tool. Chimia 54, 766–770 (2000).
Karlström, G. et al. MOLCAS: a program package for computational chemistry. Comput. Mater. Sci. 28, 222–239 (2003).
Roos, B. O. in Advances in Chemical Physics, Ab Initio Methods in Quantum Chemistry – II (ed. Lawley, K. P.) 399–446 (Wiley, 1987).
Andersson, K., Malmqvist, P.-Å., Roos, B. O., Sadlej, A. & Wolinski, K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 94, 5483–5488 (1990).
Andersson, K., Malmqvist, P.-Å. & Roos, B. O. Second-order perturbation theory with a complete active space self-consistent field reference function. J. Chem. Phys. 96, 1218–1226 (1992).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian, 2010).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Moritz, A., Cao, X. & Dolg, M. Quasirelativistic energy-consistent 5f-in-core pseudopotentials for divalent and tetravalent actinide elements. Theor. Chem. Acc. 117, 473–481 (2007).
Roos, B. O. & Malmqvist, P.-Å. Relativistic quantum chemistry: the multiconfigurational approach. Phys. Chem. Chem. Phys. 6, 2919–2927 (2004).
Douglas, N. & Kroll, N. M. Quantum electrodynamical corrections to the fine structure of helium. Ann. Phys. 82, 89–155 (1974).
Hess, B. A. Relativistic electronic-structure calculations employing a two-component no-pair formalism with external-field projection operators. Phys. Rev. A 33, 3742–3748 (1986).
Roos, B. O., Lindh, R., Malmqvist, P.-Å., Veryazov, V. & Widmark, P.-O. New relativistic ANO basis sets for actinide atoms. Chem. Phys. Lett. 409, 295–299 (2005).
Widmark, P.-O., Malmqvist, P.-Å. & Roos, B. O. Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. Theor. Chim. Acta 77, 291–306 (1990).
Roos, B. O., Lindh, R., Malmqvist, P.-Å., Veryazov, V. & Widmark, P.-O. Main group atoms and dimers studied with a new relativistic ANO basis set. J. Phys. Chem. A 108, 2851–2858 (2004).
Spivak, M., Vogiatzis, K. D., Cramer, C. J., De Graaf, C. & Gagliardi, L. Quantum chemical characterization of single molecule magnets based on uranium. J. Phys. Chem. A 121, 1726–1733 (2017).
Gaggioli, C. A. & Gagliardi, L. Theoretical investigation of plutonium-based single-molecule magnets. Inorg. Chem. 57, 8098–8105 (2018).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyser. J. Comput. Chem. 5, 580–592 (2012).
Acknowledgements
M.P. thanks J. Bendix (University of Copenhagen) for stimulating scientific discussions. Experimental work by M.S.D. was supported by the ActUsLab programme (AUL-2017-20-206) under contract with the European Commission. This work has been partially supported by the ENEN+ project, which has received funding from the Euratom research and training Work Programme 2016–2017–1 #755576 (M.S.D., O.W. and S.T.L.). Funding and support from the ENEN+ project for mobility support (A-9514681062; M.S.D.), the UK EPSRC (EP/T011289/1 and EP/M027015/1; S.T.L.), EU ERC (GoG612724; S.T.L.), COST Action CM1006 (S.T.L.), US DOE-BES Heavy Element Chemistry Program at Los Alamos National Laboratory (LANL; DE-AC52-06NA25396; C.A.P.G. and A.J.G.), LANL Laboratory Directed Research and Development program for a Distinguished J. R. Oppenheimer Postdoctoral Fellowship (LANL-LDRD 20180703PRD1; C.A.P.G.) and The University of Manchester (M.S.D., A.J.W., S.T.L.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M.S.D. planned the experiments, synthesized the complexes and acquired and interpreted their characterization data. C.A.P.G. and A.J.G. prepared compounds for electrochemical experiments and performed, analysed and interpreted the electrochemical experiments. M.P., J.-C.G., E.C. and R.C. acquired, analysed, modelled and interpreted the magnetic data. A.K. conducted the multireference calculations. A.J.W. and O.W. collected and refined the crystallographic data. S.T.L. conducted the single-reference calculations. O.W. and S.T.L. conceived the research idea, coordinated the research, analysed and interpreted all the data, and wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental and theoretical details, Supplementary Figs. 1–32 and Tables 1–8.
Supplementary Data 1
Final coordinates and energy from B3LYP calculations on NpSi3C6N4OH21.
Supplementary Data 2
Final coordinates and energy from B3LYP calculations on 3.
Supplementary Data 3
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 (all-electron).
Supplementary Data 4
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 (5d frozen core).
Supplementary Data 5
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 (6p frozen core).
Supplementary Data 6
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.84 Å.
Supplementary Data 7
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.85 Å.
Supplementary Data 8
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.86 Å.
Supplementary Data 9
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.87 Å.
Supplementary Data 10
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.88 Å.
Supplementary Data 11
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.89 Å.
Supplementary Data 12
Final coordinates and energy from a DFT single-point energy calculation on geometry-optimized 3 with Np–O distance set to 1.90 Å
Supplementary Data 13
Crystallographic information file (CIF) for 2, CCDC 2055264.
Supplementary Data 14
Crystallographic information file (CIF) for 3, CCDC 2055265.
Rights and permissions
About this article
Cite this article
Dutkiewicz, M.S., Goodwin, C.A.P., Perfetti, M. et al. A terminal neptunium(V)–mono(oxo) complex. Nat. Chem. 14, 342–349 (2022). https://doi.org/10.1038/s41557-021-00858-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00858-0
This article is cited by
-
A tetrahedral neptunium(V) complex
Nature Chemistry (2024)
-
Actinide inverse trans influence versus cooperative pushing from below and multi-center bonding
Nature Communications (2023)