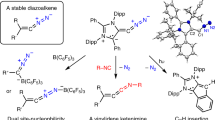
Abstract
Diazoolefins tend to be highly reactive compounds that rapidly lose dinitrogen. So far, most experimental evidence for diazoolefins is indirect, via trapping experiments. Here we show that diazoolefins are observed to form in reactions of N-heterocyclic olefins with nitrous oxide. The products benefit from resonance stabilization, which enables isolation on a preparative scale, and comprehensive characterization, which includes crystallographic analyses. N-heterocyclic diazoolefins show a strong ylidic character, with a high charge density at the carbon atom next to the diazo group. Despite the presence of terminal N2 groups, N-heterocyclic diazoolefins display a good thermal stability, which surpasses that observed for most diazoalkanes. N-heterocyclic diazoolefins can bind transition and main group metal complexes without the liberation of dinitrogen, and spectroscopic data show that they are strong electron donors. They can also undergo reactions that involve the N2 group, as evidenced by cycloaddition reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2059964 (1), 2059969 (3), 2081293 (4), 2084389 (7), 2059970 (8), 2059965 (9), 2059966 (10), 2059971 (11), 2059972 (12), 2082844 (13), 2059967 (14), 2059973 (15), 2059968 (16) and 2059974 (17). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Relevant data for this study are available within the article and its Supplementary Information. Original data files for the spectroscopic analyses can be obtained from the authors upon reasonable request.
References
Regitz, M. & Maas, G. Diazo Compounds (Academic, 1986).
Ford, A. et al. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 115, 9981–10080 (2015).
Candeias, N. R., Paterna, R. & Gois, P. M. P. Homologation reaction of ketones with diazo compounds. Chem. Rev. 116, 2937–2981 (2016).
Doyle, M. P., Duffy, R., Ratnikov, M. & Zhou, L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 110, 704–724 (2010).
Green, S. P. et al. Thermal stability and explosive hazard assessment of diazo compounds and diazo transfer reagents. Org. Process Res. Dev. 24, 67–84 (2020).
Hock, K. J. & Koenigs, R. M. The generation of diazo compounds in continuous-flow. Chem. Eur. J. 24, 10571–10583 (2018).
Knorr, R. Alkylidenecarbenes, alkylidenecarbenoids, and competing species: which Is responsible for vinylic nucleophilic substitution, [1 + 2] cycloadditions, 1.5-CH insertions, and the Fritsch–Buttenberg–Wiechell rearrangement? Chem. Rev. 104, 3795–3849 (2004).
Kirmse, W. Alkenylidenes in organic synthesis. Angew. Chem. Int. Ed. Engl. 36, 1164–1170 (1997).
Lahi, P. M. & Berson, J. A. Thermal rearrangement of an allenic diazoalkane and intermolecular capture of a diazoethene by a cyclopropene to give a common dihydropyridazine product. J. Am. Chem. Soc. 103, 7011–7012 (1981).
Ando, W., Furuhata, T. & Takata, T. A highly efficient reaction of thiobenzophenone for 1-diazoalkene. Tetrahedron Lett. 26, 4499–4500 (1985).
Munschauer, R. & Maas, G. 1,3-(C→O) silyl shift in α-diazo α-silyl ketones: cycloaddition reactions and kinetic proof for the β-siloxydiazoalkane intermediate. Angew. Chem. Int. Ed. Engl. 30, 306–308 (1991).
Munschauer, R. & Maas, G. Cycloaddition products from (1-diazo-2-oxoalkyl)silanes and cyclopropenes. A silatropic 2,3-diazabicyclo[3.1.0]hex-3-ene/1,4-dihydropyidazine equilibrium. Chem. Ber. 125, 1227–1234 (1992).
Manz, B. & Maas, G. Synthesis of 5-alkylidene-4,5-dihydro-3H-1,2,4(λ3)-diazaphospholes from α-silyl-α-diazoketones and phosphaalkenes. Tetrahedron 52, 10053–10072 (1996).
Brahms, J. C. & Dailey, W. P. Difluoropropadienone as a source of difluorovinylidene and difluorodiazoethene. J. Am. Chem. Soc. 112, 4046–4047 (1990).
Bott, K. 2,2-(N,N′-dimethyl-ethylendiamino)-ethylenediazonium-ion, ein diazonium ion mit ungewöhnlichen eigenschaften. Tetrahedron Lett. 26, 3199–3202 (1985).
Bott, K. Dialkylamino-substituierte ethylendiazoniumsalze. Chem. Ber. 120, 1867–1871 (1987).
Klein, S., Tonner, R. & Frenking, G. Carbodicarbenes and related divalent carbon(0) compounds. Chem. Eur. J. 16, 10160–10170 (2010).
Fustier-Bouitignon, M., Nebra, N. & Mézailles, N. Geminal dianions stabilized by main group elements. Chem. Rev. 119, 8555–8700 (2019).
Frenking, G. & Tonner, R. Carbodicarbenes–divalent carbon(0) compounds exhibiting carbon–carbon donor–acceptor bonds. WIREs Comput. Mol. Sci. 1, 869–878 (2011).
Antoni, P. W., Golz, C., Holstein, J. J., Pantazis, D. & Hansmann, M. M. Isolation and reactivity of an elusive diazoalkene. Nat. Chem. 13, 587–593 (2021).
Roy, M. M. D. & Rivard, E. Pushing chemical boundaries with N-heterocyclic olefins (NHOs): from catalysis to main group element chemistry. Acc. Chem. Res. 50, 2017–2025 (2017).
Crocker, R. D. & Nguyen, T. V. The resurgence of the highly ylidic N-heterocyclic olefins as a new class of organocatalysts. Chem. Eur. J. 22, 2208–2213 (2016).
Naumann, S. Synthesis, properties & applications of N-heterocyclic olefins in catalysis. Chem. Commun. 55, 11658–11670 (2019).
Severin, K. Synthetic chemistry with nitrous oxide. Chem. Soc. Rev. 44, 6375–6386 (2015).
Eymann, L. Y. M. et al. Synthesis of organic super-electron-donors by reaction of nitrous oxide with N-heterocyclic olefins. J. Am. Chem. Soc. 141, 17112–17116 (2019).
Allen, F. H. Bond lengths relationships in diazo and diazonium compounds. Acta Cryst. B 51, 378–381 (1995).
Al-Rafia, S. M. I. et al. Intercepting low oxidation sate main group hydrides with a nucleophilic N-heterocyclic olefin. Chem. Commun. 47, 6987–6989 (2011).
Gruber, M., Bauer, W., Maid, H., Schöll, K. & Tykwinski, R. R. Synthetic and NMR studies on hexaphenylcarbodiphosphorane (Ph3P=C=PPh3). Inorg. Chim. Acta 468, 152–158 (2017).
Frisch, M. et al. Gaussian 16, Revision A.03 (Gaussian, Inc., 2016).
Petz, W. Addition compounds between carbones, CL2, and main group Lewis acids: a new glance at old and new compounds. Coord. Chem. Rev. 291, 1–27 (2015).
Zhao, L., Chai, C., Petz, W. & Frenking, G. Carbones and carbon atom as ligands in transition metal complexes. Molecules 25, 4943 (2020).
Wiberg, K. B. Application of the Pople–Santry–Segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968).
Glendening, E. D. & Weinhold, F. Natural resonance theory: II. Natural bond order and valency. J. Comput. Chem. 19, 610–627 (1998).
Glendening, E. D. & Weinhold, F. Natural resonance theory: I. General formalism. J. Comput. Chem. 19, 593–609 (1998).
Glendening, E. D., Badenhoop, J. & Weinhold, F. Natural resonance theory: III. Chemical applications. J. Comput. Chem. 19, 628–646 (1998).
Yang, Z., Stivanin, M. L., Jurberg, I. D. & Koenigs, R. M. Visible light-promoted reactions with diazo compounds: a mild and practical strategy towards free carbene intermediates. Chem. Soc. Rev. 49, 6833–6847 (2020).
Tskhovrebov, A. G., Vuichoud, B., Solari, E., Scopelliti, R. & Severin, K. Adducts of nitrous oxide and N-heterocyclic carbenes: syntheses, structures, and reactivity. J. Am. Chem. Soc. 135, 9486–9492 (2013).
Eymann, L. Y. M., Scopelliti, R., Tirani, F. F. & Severin, K. Synthesis of azo dyes from mesoionic carbenes and nitrous oxide. Chem. Eur. J. 24, 7957–7963 (2018).
Liu, S.-k, Shih, W.-C., Chen, W.-C. & Ong, T.-G. Carbodicarbenes and their captodative behavior in catalysis. ChemCatChem 10, 1483–1498 (2018).
Dyker, C. A., Lavallo, V., Donnadieu, B. & Bertrand, G. Synthesis of an extremely bent acyclic allene (a ‘carbodicarbene’): a strong donor ligand. Angew. Chem. Int. Ed. 47, 3206–3209 (2008).
Chen, W.-C., Hsu, Y.-C., Lee, C.-Y., Yap, G. P. A. & Ong, T.-G. Synthetic modification of acyclic bent allenes (carbodicarbenes) and further studies on their structural implications and reactivities. Organometallics 32, 2435–2442 (2013).
Chen, W.-C. et al. Expanding the ligand framework diversity of carbodicarbenes and direct detection of boron activation in the methylation of amines with CO2. Angew. Chem. Int. Ed. 54, 15207–15212 (2015).
Chen, W.-C. et al. The elusive three-coordinate dicationic hydrido boron complex. J. Am. Chem. Soc. 136, 914–917 (2014).
Dröge, T. & Glorius, F. The measure of all rings—N-heterocyclic carbenes. Angew. Chem. Int. Ed. 49, 6940–6952 (2010).
Acknowledgements
This work was supported by funding from the EPFL. We thank A. Gitlina for the infrared spectroscopy measurements, W. Feuerstein for help with the synthesis of 1, C. Berton for help with NMR analyses and D. Ortiz for help with mass spectrometry.
Author information
Authors and Affiliations
Contributions
P.V. and K.S. designed the experiments, P.V. performed the synthetic experiments and analysed the data, Z.D. performed the computational analysis, R.S. and F.F.-T. collected and processed the X-ray data, and P.V., Z.D. and K.S. co-wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Gabriela Borosky, Tiow-Gan Ong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, analytical data including copies of NMR spectra, and details regarding the crystallographic and computational analyses.
Supplementary Data 1
Crystallographic data for compound 1; CCDC reference 2059964.
Supplementary Data 2
Crystallographic data for compound 3; CCDC reference 2059969.
Supplementary Data 3
Crystallographic data for compound 4; CCDC reference 2081293.
Supplementary Data 4
Crystallographic data for compound 7; CCDC reference 2084389.
Supplementary Data 5
Crystallographic data for compound 8; CCDC reference 2059970.
Supplementary Data 6
Crystallographic data for compound 9; CCDC reference 2059965.
Supplementary Data 7
Crystallographic data for compound 10; CCDC reference 2059966.
Supplementary Data 8
Crystallographic data for compound 11; CCDC reference 2059971.
Supplementary Data 9
Crystallographic data for compound 12; CCDC reference 2059972.
Supplementary Data 10
Crystallographic data for compound 13; CCDC reference 2082844.
Supplementary Data 11
Crystallographic data for compound 14; CCDC reference 2059967.
Supplementary Data 12
Crystallographic data for compound 15; CCDC reference 2059973.
Supplementary Data 13
Crystallographic data for compound 16; CCDC reference 2059968.
Supplementary Data 14
Crystallographic data for compound 17; CCDC reference 2059974.
Rights and permissions
About this article
Cite this article
Varava, P., Dong, Z., Scopelliti, R. et al. Isolation and characterization of diazoolefins. Nat. Chem. 13, 1055–1060 (2021). https://doi.org/10.1038/s41557-021-00790-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00790-3
This article is cited by
-
Arenes participate in 1,3-dipolar cycloaddition with in situ-generated diazoalkenes
Nature Chemistry (2023)
-
New chiral N-heterocyclic olefin bifunctional organocatalysis in α-functionalization of β-ketoesters
Science China Chemistry (2023)
-
More details on diazoolefins
Nature Chemistry (2021)