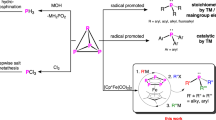
Abstract
In contrast to the well-established transition-metal-mediated activation of white phosphorus (P4), the metal-free direct functionalization of P4 has remained rare. The conversion of P4 into a reactive zero-valent diphosphorus compound (P2) has proven challenging to carry out without relying on metal reactivity. Herein, we describe the facile degradation of P4 mediated by two divalent silicon atoms in a bis(silylene) scaffold, resulting in a silylene-stabilized zero-valent P2 complex. The presence of two lone pairs of electrons on each P atom in the silylene-stabilized P2 complex enables a rich reactivity towards small molecules; reaction of the P2 species with CO2, water or a borane leads to the formation of P–C, P–H or P–B bonds, respectively. Notably, the P2 complex also serves as a single phosphorus anion (P−) transfer reagent towards metal carbonyls and a chlorogermylene compound, leading to the synthetically valuable phosphaketenide (PCO−) ligand and a phosphinidene germylene complex, respectively.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1978612 (2), 1978615 (3), 1978613 (4), 1978616 (5), 1978614 (6), 1978617 (7), 1978618 (8), 1978619 (9), 1978620 (10). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available within the Article, its Supplementary Information and from the corresponding author upon reasonable request.
References
Corbridge, D. E. C. Phosphorus: an Outline of its Chemistry, Biochemistry, and Technology 5th edn (Elsevier, 1995).
Peruzzini, M., Gonsalvi, L. & Romerosa, A. Coordination chemistry and functionalization of white phosphorus via transition metal complexes. Chem. Soc. Rev. 34, 1038–1047 (2005).
Cummins, C. C. Terminal, anionic carbide, nitride, and phosphide transition-metal complexes as synthetic entries to low-coordinate phosphorus derivatives. Angew. Chem. Int. Ed. 45, 862–870 (2006).
Cossairt, B. M., Piro, N. A. & Cummins, C. C. Early-transition-metal-mediated activation and transformation of white phosphorus. Chem. Rev. 110, 4164–4177 (2010).
Caporali, M., Gonsalvi, L., Rossin, A. & Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 110, 4178–4235 (2010).
Figueroa, J. S. & Cummins, C. C. A niobaziridine hydride system for white phosphorus or dinitrogen activation and N- or P-atom transfer. Dalton Trans. 2161–2168 (2006).
Piro, N. A., Figueroa, J. S., McKellar, J. T. & Cummins, C. C. Triple-bond reactivity of diphosphorus molecules. Science 313, 1276–1279 (2006).
Cossairt, B. M. & Cummins, C. C. A niobium-mediated cycle producing phosphorus-rich organic molecules from white phosphorus (P4) through activation, functionalization, and transfer reactions. Angew. Chem. Int. Ed. 47, 8863–8866 (2008).
Tofan, D. & Cummins, C. C. Photochemical incorporation of diphosphorus units into organic molecules. Angew. Chem. Int. Ed. 49, 7516–7518 (2010).
Scheer, M., Balázs, G. & Seitz, A. P4 activation by main group elements and compounds. Chem. Rev. 110, 4236–4256 (2010).
Giffin, N. A. & Masuda, J. D. Reactivity of white phosphorus with compounds of the p-block. Coord. Chem. Rev. 255, 1342–1359 (2011).
Khan, S., Sen, S. S. & Roesky, H. W. Activation of phosphorus by group 14 elements in low oxidation states. Chem. Commun. 48, 2169–2179 (2012).
Holthausen, M. H. & Weigand, J. J. The chemistry of cationic polyphosphorus cages–syntheses, structure and reactivity. Chem. Soc. Rev. 43, 6639–6657 (2014).
Borger, J. E., Ehlers, A. W., Slootweg, J. C. & Lammertsma, K. Functionalization of P4 through direct P−C bond formation. Chem. Eur. J. 23, 11738–11746 (2017).
Dorsey, C. L., Squires, B. M. & Hudnall, T. W. Isolation of a neutral P8 cluster by [2+2] cycloaddition of a diphosphene facilitated by carbene activation of white phosphorus. Angew. Chem. Int. Ed. 52, 4462–4465 (2013).
Rottschäfer, D., Blomeyer, S., Neumann, B., Stammler, H.-G. & Ghadwal, R. S. Direct functionalization of white phosphorus with anionic dicarbenes and mesoionic carbenes: facile access to 1,2,3-triphosphol-2-ides. Chem. Sci. 10, 11078–11085 (2019).
Peng, Y. et al. [{HC(CMeNAr)2}2Al2P4] (Ar = 2,6-iPr2C6H3): a reduction to a formal {P4}4− charged species. Angew. Chem. Int. Ed. 43, 3443–3445 (2004).
Fox, A. R., Wright, R. J., Rivard, E. & Power, P. P. Tl2[Aryl2P4]: a thallium complexed diaryltetraphosphabutadienediide and its two-electron oxidation to a diaryltetraphosphabicyclobutane, Aryl2P4. Angew. Chem. Int. Ed. 44, 7729–7733 (2005).
Back, O., Kuchenbeiser, G., Donnadieu, B. & Bertrand, G. Nonmetal-mediated fragmentation of P4: isolation of P1 and P2 bis(carbene) adducts. Angew. Chem. Int. Ed. 48, 5530–5533 (2009).
Masuda, J. D., Schoeller, W. W., Donnadieu, B. & Bertrand, G. Carbene activation of P4 and subsequent derivatization. Angew. Chem. Int. Ed. 46, 7052–7055 (2007).
Martin, C. D., Weinstein, C. M., Moore, C. E., Rheingold, A. L. & Bertrand, G. Exploring the reactivity of white phosphorus with electrophilic carbenes: synthesis of a P4 cage and P8 clusters. Chem. Commun. 49, 4486–4488 (2013).
Masuda, J. D., Schoeller, W. W., Donnadieu, B. & Bertrand, G. NHC-mediated aggregation of P4: isolation of a P12 cluster. J. Am. Chem. Soc. 129, 14180–14181 (2007).
Xiong, Y., Yao, S., Brym, M. & Driess, M. Consecutive insertion of a silylene into the P4 tetrahedron: facile access to strained SiP4 and Si2P4 cage compounds. Angew. Chem. Int. Ed. 46, 4511–4513 (2007).
Alvarado-Beltran, I., Baceiredo, A., Saffon-Merceron, N., Branchadell, V. & Kato, T. Cyclic amino(ylide) silylene: a stable heterocyclic silylene with strongly electron-donating character. Angew. Chem. Int. Ed. 55, 16141–16144 (2016).
Sen, S. S. et al. Zwitterionic Si-C-Si-P and Si-P-Si-P four-membered rings with two-coordinate phosphorus atoms. Angew. Chem. Int. Ed. 50, 2322–2325 (2011).
Khan, S., Michel, R., Sen, S. S., Roesky, H. W. & Stalke, D. A P4 chain and cage from silylene-activated white phosphorus. Angew. Chem. Int. Ed. 50, 11786–11789 (2011).
Driess, M., Fanta, A. D., Powell, D. R. & West, R. Synthesis, characterization, and complexation of an unusual P2Si2 bicyclobutane with butterfly-structure: 2,2,4,4-tetramesityl-1,3-diphospha-2,4-disilabicyclo[1.1.0]butane. Angew. Chem. lnt. Ed. Engl. 28, 1038–1040 (1989).
Wang, Y. et al. Carbene-stabilized diphosphorus. J. Am. Chem. Soc. 130, 14970–14971 (2008).
Bock, H. & Müller, H. Gas-phase reactions. 44. The P4 ↔ 2P2 equilibrium visualized. Inorg. Chem. 23, 4365–4368 (1984).
Kornath, A., Kaufmann, A. & Torheyden, M. Raman spectroscopic studies on matrix isolated phosphorus molecules P4 and P2. J. Chem. Phys. 116, 3323–3326 (2002).
Rottschäfer, D. et al. A phosphorus analogue of p-quinodimethane with a planar P4 ring: a metal-free diphosphorus source. Chem. Eur. J. 25, 3244–3247 (2019).
Wang, Y., Kostenko, A., Yao, S. & Driess, M. Divalent silicon-assisted activation of dihydrogen in a bis(N-heterocyclic silylene)xanthene nickel(0) complex for efficient catalytic hydrogenation of olefin. J. Am. Chem. Soc. 139, 13499–13506 (2017).
Wang, Y. et al. Silicon-mediated selective homo- and heterocoupling of carbon monoxide. J. Am. Chem. Soc. 141, 626–634 (2019).
Wang, Y., Karni, M., Yao, S., Apeloig, Y. & Driess, M. An isolable bis(silylene)-stabilized germylone and its reactivity. J. Am. Chem. Soc. 141, 1655–1664 (2019).
Wang, Y. et al. Synthesis of an isolable bis(silylene)-stabilized silylone and its reactivity toward small gaseous molecules. J. Am. Chem. Soc. 141, 12916–12927 (2019).
Tondreau, A. M., Benkő, Z., Harmerb, J. R. & Grützmacher, H. Sodium phosphaethynolate, Na(OCP), as a “P” transfer reagent for the synthesis of N-heterocyclic carbene supported P3 and PAsP radicals. Chem. Sci. 5, 1545–1554 (2014).
Heift, D., Benkő, Z. & Grützmacher, H. Redox-triggered reversible interconversion of a monocyclic and a bicyclic phosphorus heterocycle. Angew. Chem. Int. Ed. 53, 6757–6761 (2014).
Suter, R. et al. 2,4,6-Tri(hydroxy)-1,3,5-triphosphinine, P3C3(OH)3: the phosphorus analogue of cyanuric acid. Angew. Chem. Int. Ed. 56, 1356–1360 (2017).
Jupp, A. R. & Goicoechea, J. M. Phosphinecarboxamide: a phosphorus-containing analogue of urea and stable primary phosphine. J. Am. Chem. Soc. 135, 19131–19134 (2013).
Goicoechea, J. M. & Grützmacher, H. The chemistry of the 2-phosphaethynolate anion. Angew. Chem. Int. Ed. 57, 16968–16994 (2018).
Puschmann, F. F. et al. Phosphination of carbon monoxide: a simple synthesis of sodium phosphaethynolate (NaOCP). Angew. Chem. Int. Ed. 50, 8420–8423 (2011).
Suter, R., Benkö, Z., Bispinghoff, M. & Grützmacher., H. Annulated 1,3,4-azadiphospholides: heterocycles with widely tunable optical properties. Angew. Chem. Int. Ed. 56, 11226–11231 (2017).
Benedek, Z. & Szilvási, T. Can low-valent silicon compounds be better transition metal ligands than phosphines and NHCs? RSC Adv. 5, 5077–5086 (2015).
Hersh, W. H. False AA′X spin−spin coupling systems in 13C NMR: examples involving phosphorus and a 20-year-old mystery in off-resonance decoupling. J. Chem. Educ. 74, 1485–1488 (1997).
Driess, M., Rell, S., Pritzkow, H. & Janoschek, R. R2Si=P−SiR2F: 1,3-sigmatropic migration of fluorine in a 2-phospha-l,3-disilaallyl derivative capable of conjugation and its conversion to phosphadisilacyclopropanes. Angew. Chem. Int. Ed. Engl. 36, 1326–1329 (1997).
Lee, V. Y. A., Kawai, M., Sekiguchi, A., Ranaivonjatovo, H. & Escudié, J. A “push–pull” phosphasilene and phosphagermene and their anion-radicals. Organometallics 28, 4262–4265 (2009).
Back, O., Donnadieu, B., Parameswaran, P., Frenking, G. & Bertrand, G. Isolation of crystalline carbene-stabilized P2-radical cations and P2-dications. Nat. Chem. 2, 369–373 (2010).
Szilvási, T. & Veszprémi, T. Why do N-heterocyclic carbenes and silylenes activate white phosphorus differently? Struct. Chem. 26, 1335–1342 (2015).
Szilvási, T. & Veszprémi, T. On the mechanism of the reaction of white phosphorus with silylenes. Dalton Trans. 40, 7193–7200 (2011).
Sakakura, T., Choi, J.-C. & Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 107, 2365–2387 (2007).
Mömming, C. M. et al. Reversible metal-free carbon dioxide binding by frustrated Lewis pairs. Angew. Chem. Int. Ed. 48, 6643–6646 (2009).
Ellis, B. D., Dyker, C. A., Decken, A. & Macdonald, C. L. B. The synthesis, characterisation and electronic structure of N-heterocyclic carbene adducts of PI cations. Chem. Commun., 1965–1967 (2005)
Ellis, B. D. & Macdonald, C. L. B. Phosphorus(I) iodide: a versatile metathesis reagent for the synthesis of low oxidation state phosphorus compounds. Inorg. Chem. 45, 6864–6874 (2006).
Yao, S. et al. Facile access to NaOC≡As and its use as an arsenic source to form germylidenylarsinidene complexes. Angew. Chem. Int. Ed. 56, 7465–7469 (2017).
Yao, S., Xiong, Y., Szilvási, T., Grützmacher, H. & Driess, M. From a phosphaketenyl-functionalized germylene to 1,3-digerma-2,4-diphosphacyclobutadiene. Angew. Chem. Int. Ed. 55, 4781–4785 (2016).
Buhling, A. et al. Novel amphiphilic diphosphines: synthesis, X-ray structure, rhodium complexes, use in hydroformylation, and rhodium recycling. Organometallics 16, 3027–3037 (1997).
Sen, S. S., Roesky, H. W., Stern, D., Henn, J. & Stalke, D. High yield access to silylene RSiCl (R = PhC(NtBu)2) and its reactivity toward alkyne: synthesis of stable disilacyclobutene. J. Am. Chem. Soc. 132, 1123–1126 (2010).
Nagendran, S. et al. RGe(I)Ge(I)R compound (R = PhC(NtBu)2) with a Ge−Ge single bond and a comparison with the gauche conformation of hydrazine. Organometallics 27, 5459–5463 (2008).
Acknowledgements
We thank P. Nixdorf for assistance with the X-ray diffraction measurements. This work was financially supported by the Deutsche Forschungsgemeinschaft (DR 226/19-2) (Germany’s Excellence Strategy−EXC 2008/1-390540038 (UniSysCat)). Y.W. acknowledges financial support from the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
M.D. and Y.W. conceived and designed the experiments. Y.W. carried out the synthetic experiments and analysed the experimental data. T.S. performed the theoretical calculations. Y.W. and S.Y. conducted the crystallographic studies. M.D., Y.W. and T.S. wrote the manuscript. M.D. supervised the study. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Natural Resonance Theory analysis for the surrogates of A, B and 2.
a, Natural Resonance Theory analysis for surrogate of A. Bulky substituents of the cAAC ligand are replaced by hydrogens. Resonance structures with more than 1 weight percent is shown. b, Natural Resonance Theory analysis for surrogate of B. Bulky substituents of the NHC ligand are replaced by hydrogens. No covalent reference structure was found thus NRT analysis was not successful and these results could implicitly point to the importance of donor-acceptor reference structure. c, Natural Resonance Theory analysis for surrogate of 2. Ph and tBu substituents of the amidinate ligand are replaced by hydrogens and the xanthene backbone were replaced by two methyl groups. No covalent reference structure was found thus NRT analysis was not successful and these results could implicitly point to the importance of donor-acceptor reference structure.
Extended Data Fig. 2 DFT-derived mechanism of P4 activation by 1 leading to 2.
In the initial step, the lone pair of electrons at one silylene group induce the cage-opening in P4 to give the intermediate D. Then, the other silylene moiety in 1 interacts with the P = P bond affording compound E, which can further react with one molecule of 1 to form the branched intermediate F. As bis(silylene) 1 involves another reactive silylene moiety in close proximity, the reaction can go further to fragment the P4 moiety resulting in the bis(NHSi)-supported P2 complex 2.
Supplementary information
Supplementary Information
General considerations, synthetic procedures, compound characterization data including spectroscopic and analytical data for all new compounds, NMR spectra, single-crystal X-ray crystallographic data, computational details containing Cartesian coordinates of computational structures, Supplementary Figs. 1–54, Tables 1–45 and references 1–11.
Supplementary Data 1
CIF for compound 2; CCDC reference: 1978612.
Supplementary Data 2
CIF for compound 3; CCDC reference: 1978615.
Supplementary Data 3
CIF for compound 4; CCDC reference: 1978613.
Supplementary Data 4
CIF for compound 5; CCDC reference: 1978616.
Supplementary Data 5
CIF for compound 6; CCDC reference: 1978614.
Supplementary Data 6
CIF for compound 7; CCDC reference: 1978617.
Supplementary Data 7
CIF for compound 8; CCDC reference: 1978618.
Supplementary Data 8
CIF for compound 9; CCDC reference: 1978619.
Supplementary Data 9
CIF for compound 10; CCDC reference: 1978620.
Rights and permissions
About this article
Cite this article
Wang, Y., Szilvási, T., Yao, S. et al. A bis(silylene)-stabilized diphosphorus compound and its reactivity as a monophosphorus anion transfer reagent. Nat. Chem. 12, 801–807 (2020). https://doi.org/10.1038/s41557-020-0518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0518-0
This article is cited by
-
A silylene-stabilized ditin(0) complex and its conversion to methylditin cation and distannavinylidene
Nature Communications (2023)
-
A class of non-aromatic 1,3-disilapyrroles acting as stable organosilicon-based triplet diradicals
Nature Synthesis (2023)
-
A convenient P– source
Nature Chemistry (2020)