Abstract
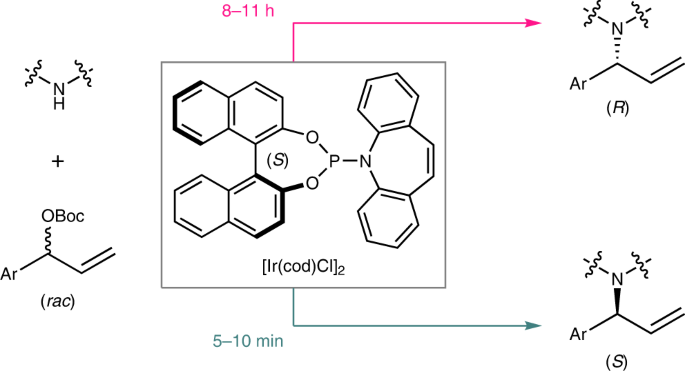
The preparation of both enantiomers of chiral molecules is among the most fundamental tasks in organic synthesis, medicinal chemistry and materials science. Achieving this goal typically requires reversing the absolute configuration of the chiral component employed in the reaction system that is being used. The task becomes challenging when the natural source of the chiral component is not available in both configurations. Herein, we report a time-dependent enantiodivergent synthesis, in which an Ir-catalysed allylic substitution reaction uses one catalyst sequentially to promote two kinetic resolution reactions, enabling the synthesis of both enantiomers of the product using the same enantiomer of a chiral catalyst. The appropriate permutation of individual reaction rates is essential for the isolation of the chiral products in opposite configurations with high enantiopurity when quenched at different reaction times. This work provides an alternative solution for the preparation of both enantiomers of chiral molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published Article and Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1917286 (5). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Change history
12 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41557-020-0546-9
References
Ariëns, E. J. Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur. J. Clin. Pharmacol. 26, 663–668 (1984).
McConathy, J. & Owens, M. J. Stereochemistry in drug action. Prim. Care Companion J. Clin. Psychiatry 5, 70–73 (2003).
Carreira, E. M. & Yamamoto, H. (eds) Comprehensive Chirality (Elsevier, 2012).
Wang, Y., Xu, J., Wang, Y. & Chen, H. Emerging chirality in nanoscience. Chem. Soc. Rev. 42, 2930–2962 (2013).
Liu, M., Zhang, L. & Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 115, 7304–7397 (2015).
Brill, Z. G., Condakes, M. L., Ting, C. P. & Maimone, T. J. Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem. Rev. 117, 11753–11795 (2017).
Gnas, Y. & Glorius, F. Chiral auxiliaries – principles and recent applications. Synthesis 2006, 1899–1930 (2006).
Paquette, L. A. (ed.) Handbook of Reagents for Organic Synthesis: Chiral Reagents for Asymmetric Synthesis (Wiley, 2003).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis (Springer, 1999).
Yoon, T. P. & Jacobsen, E. N. Privileged chiral catalysts. Science 299, 1691–1693 (2003).
Zhou, Q.-L. (ed.) Privileged Chiral Ligands and Catalysts (Wiley, 2011).
Blaser, H.-U. & Schmidt, E. (eds) Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions (Wiley, 2004).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Oliveira, M. T., Luparia, M., Audisio, D. & Maulide, N. Dual catalysis becomes diastereodivergent. Angew. Chem. Int. Ed. 52, 13149–13152 (2013).
Shi, S.-L., Wong, Z. L. & Buchwald, S. L. Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols. Nature 532, 353–356 (2016).
Krautwald, S. & Carreira, E. M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 139, 5627–5639 (2017).
Sibi, M. P. & Liu, M. Reversal of stereochemistry in enantioselective transformations. Can they be planned or are they just accidental? Curr. Org. Chem. 5, 719–755 (2001).
Bartók, M. Unexpected inversions in asymmetric reactions: reactions with chiral metal complexes, chiral organocatalysts, and heterogeneous chiral catalysts. Chem. Rev. 110, 1663–1705 (2010).
Beletskaya, I. P., Nájera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Yamaguchi, S., Mosher, H. S. & Pohland, A. Reversal in stereoselectivity depending upon the age of a chiral lithium alkoxyaluminohydride reducing reagent. J. Am. Chem. Soc. 94, 9254–9255 (1972).
Zhou, J. & Tang, Y. Enantioselective Friedel–Crafts reaction of indoles with arylidene malonates catalyzed by iPr-bisoxazoline-Cu(OTf)2. Chem. Commun. 432–433 (2004).
Trost, B. M., Fettes, A. & Shireman, B. T. Direct catalytic asymmetric aldol additions of methyl ynones. Spontaneous reversal in the sense of enantioinduction. J. Am. Chem. Soc. 126, 2660–2661 (2004).
Kagan, H. B. & Fiaud, J. C. Kinetic resolution. Top. Stereochem. 18, 249–330 (1988).
Keith, J. M., Larrow, J. F. & Jacobsen, E. N. Practical considerations in kinetic resolution reactions. Adv. Synth. Catal. 343, 5–26 (2001).
Vedejs, E. & Jure, M. Efficiency in nonenzymatic kinetic resolution. Angew. Chem. Int. Ed. 44, 3974–4001 (2005).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Vedejs, E. & Chen, X. Parallel kinetic resolution. J. Am. Chem. Soc. 119, 2584–2585 (1997).
Wu, B., Parquette, J. R. & RajanBabu, T. V. Regiodivergent ring opening of chiral aziridines. Science 326, 1662 (2009).
Miller, L. C. & Sarpong, R. Divergent reactions on racemic mixtures. Chem. Soc. Rev. 40, 4550–4562 (2011).
Kreituss, I. & Bode, J. W. Flow chemistry and polymer-supported pseudoenantiomeric acylating agents enable parallel kinetic resolution of chiral saturated N-heterocycles. Nature Chem. 9, 446–452 (2017).
Pape, A. R., Kaliappan, K. P. & Kündig, E. P. Transition-metal-mediated dearomatization reactions. Chem. Rev. 100, 2917–2940 (2000).
Roche, S. P. & Porco, J. A. Jr. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 50, 4068–4093 (2011).
Zhuo, C.-X., Zhang, W. & You, S.-L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 51, 12662–12686 (2012).
Zhuo, C.-X., Zheng, C. & You, S.-L. Transition-metal-catalyzed asymmetric allylic dearomatization reactions. Acc. Chem. Res. 47, 2558–2573 (2014).
Zheng, C. & You, S.-L. Catalytic asymmetric dearomatization by transition-metal catalysis: a method for transformations of aromatic compounds. Chem 1, 830–857 (2016).
Yang, Z.-P., Jiang, R., Zheng, C. & You, S.-L. Iridium-catalyzed intramolecular asymmetric allylic alkylation of hydroxyquinolines: simultaneous weakening of the aromaticity of two consecutive aromatic rings. J. Am. Chem. Soc. 140, 3114–3119 (2018).
Trost, B. M. & Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 103, 2921–2944 (2003).
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 47, 258–297 (2008).
Cheng, Q. et al. Iridium-catalyzed asymmetric allylic substitution reactions. Chem. Rev. 119, 1855–1969 (2019).
Zhao, X., Liu, D., Guo, H., Liu, Y. & Zhang, W. C–N bond cleavage of allylic amines via hydrogen bond activation with alcohol solvents in Pd-catalyzed allylic alkylation of carbonyl compounds. J. Am. Chem. Soc. 133, 19354–19357 (2011).
Schreiber, S. L., Schreiber, T. S. & Smith, D. B. Reactions that proceed with a combination of enantiotopic group and diastereotopic face selectivity can deliver products with very high enantiomeric excess: experimental support of a mathematical model. J. Am. Chem. Soc. 109, 1525–1529 (1987).
Rakels, J. L. L., Wolff, A., Straathof, A. J. J. & Heijnen, J. J. Sequential kinetic resolution by two enantioselective enzymes. Biocatalysis 9, 31–47 (1994).
Kroutil, W., Kleewein, A. & Faber, K. A computer program for analysis, simulation and optimization of asymmetric catalytic processes proceeding through two consecutive steps. Type 2: sequential kinetic resolutions. Tetrahedron Asymmetry 8, 3263–3274 (1997).
Elenkov, M. M., Tang, L., Hauer, B. & Janssen, D. B. Sequential kinetic resolution catalyzed by halohydrin dehalogenase. Org. Lett. 8, 4227–4229 (2006).
Harrer, S., Greenhalgh, M. D., Neyyappadath, R. M. & Smith, A. D. Isothiourea-catalysed sequential kinetic resolution of acyclic (±)-1,2-diols. Synlett 30, 1555–1560 (2019).
Acknowledgements
This paper is dedicated to H. N. C. Wong on the occasion of his 70th birthday. Financial support for this work was provided by the National Key R&D Program of China (2016YFA0202900), National Natural Science Foundation of China (21821002, 91856201) and Chinese Academy of Sciences (XDB20000000, QYZDY-SSW-SLH012). S.-L.Y. acknowledges the support from the Tencent Foundation through the XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
H.-F.T. discovered the sequential KR reactions, optimized the conditions and evaluated the scope of the reaction; H.-F.T., P.Y. and C.Z. performed the mechanistic studies; and H.-F.T., P.Y. and Z.-H.L. conducted the derivation of the products. S.-L.Y. conceived and supervised the project. C.Z. wrote the manuscript with revisions suggested by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 3 Time-dependent enantiodivergent synthesis via Ir-catalyzed asymmetric allylic substitution with N-methyl aniline.
a, Optimal results for the synthesis of both enantiomers of 10. b, The course of the e.e. value of 10 over the reaction time. Reaction conditions: N-methyl aniline (0.1 mmol, 1.0 equiv.), (rac)-2a (2.0 equiv.), [Ir(cod)Cl]2 (1.5 mol%), (S)-L1 (6 mol%), 3,5-Cl2C6H3CO2H (10 mol%) in MeOH (0.1 M) at 40 °C.
Extended Data Fig. 4 Time-dependent enantiodivergent synthesis via Ir-catalyzed asymmetric allylic substitution with N-allyl aniline.
a, Optimal results for the synthesis of both enantiomers of 11. b, The course of the e.e. value of 11 over the reaction time. Reaction conditions: N-allyl aniline (0.2 mmol, 1.0 equiv.), (rac)-2a (2.0 equiv.), [Ir(cod)Cl]2 (1.5 mol%), (S)-L1 (6 mol%), 3,5-Cl2C6H3CO2H (10 mol%) in MeOH (0.1 M) at 40 °C.
Supplementary information
Supplementary Information
Details of optimizations of reaction conditions, product derivations, mechanistic studies and full characterization data of all new compounds. Supplementary Figs. 1–3 and Tables 1–9.
Rights and permissions
About this article
Cite this article
Tu, HF., Yang, P., Lin, ZH. et al. Time-dependent enantiodivergent synthesis via sequential kinetic resolution. Nat. Chem. 12, 838–844 (2020). https://doi.org/10.1038/s41557-020-0489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0489-1
This article is cited by
-
Real-time monitoring of reaction stereochemistry through single-molecule observations of chirality-induced spin selectivity
Nature Chemistry (2023)
-
Rhodium-catalyzed enantioselective and diastereodivergent access to diaxially chiral heterocycles
Nature Communications (2023)
-
Detecting the single chiral molecule during the reaction
Science China Materials (2023)



