Abstract
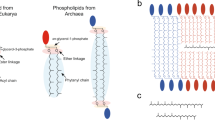
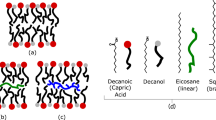
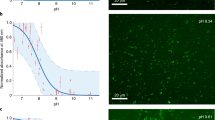
All living organisms synthesize phospholipids as the primary constituent of their cell membranes. Enzymatic synthesis of diacylphospholipids requires preexisting membrane-embedded enzymes. This limitation has led to models of early life in which the first cells used simpler types of membrane building blocks and has hampered integration of phospholipid synthesis into artificial cells. Here we demonstrate an enzyme-free synthesis of natural diacylphospholipids by transacylation in water, which is enabled by a combination of ion pairing and self-assembly between lysophospholipids and acyl donors. A variety of membrane-forming cellular phospholipids have been obtained in high yields. Membrane formation takes place in water from natural alkaline sources such as soda lakes and hydrothermal oceanic vents. When formed vesicles are transferred to more acidic solutions, electrochemical proton gradients are spontaneously established and maintained. This high-yielding non-enzymatic synthesis of natural phospholipids in water opens up new routes for lipid synthesis in artificial cells and sheds light on the origin and evolution of cellular membranes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information.
References
Jackowski, S, Cronan, J. E. Jr & Rock, C. O. Biochemistry of Lipids, Lipoproteins and Membranes Vol. 20, 80–81 (Elsevier, 1991).
Lands, W. E. M. Metabolism of glycerolipides: a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231, 883–888 (1958).
Schmidli, P. K., Schurtenberger, P. & Luisi, P. L. Liposome-mediated enzymatic synthesis of phosphatidylcholine as an approach to self-replicating liposomes. J. Am. Chem. Soc. 113, 8127–8130 (1991).
Deamer, D. W. & Boatman, D. E. An enzymatically driven membrane reconstitution from solubilized components. J. Cell Biol. 84, 461–467 (1980).
Morris-Natschke, S. L. et al. Synthesis of phosphocholine and quaternary amine ether lipids and evaluation of in vitro antineoplastic activity. J. Med. Chem. 36, 2018–2025 (1993).
Harayama, T. et al. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metabol. 20, 295–305 (2014).
Hargreaves, W. R., Mulvihill, S. J. & Deamer, D. W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 266, 78–80 (1977).
Fernandez-Garcia, C. & Powner, M. W. Selective acylation of nucleosides, nucleotides, and glycerol-3-phosphocholine in water. Synlett 28, 78–83 (2017).
Bonfio, C. et al. Length-selective synthesis of acylglycerol-phosphates through energy-dissipative cycling. J. Am. Chem. Soc. 141, 3934–3939 (2019).
Szostak, J. W., Bartel, D. P. & Luisi, P. L. Synthesizing life. Nature 409, 387–390 (2001).
Budin, I. & Szostak, J. W. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Natl Acad. Sci. USA 108, 5249–5254 (2011).
de Ouve, C. The beginnings of life on earth. American Scientist 83, 428–437 (1995).
Ejsinga, C. S. et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl Acad. Sci. USA 106, 2136–2141 (2009).
Brea, R. J., Cole, C. M. & Devaraj, N. K. In situ vesicle formation by native chemical ligation. Angew. Chem. Int. Ed. 53, 14102–14105 (2014).
Bender, M. L. Mechanisms of catalysis of nucleophilic reactions of carboxylic acid derivatives. Chem. Rev. 60, 53–113 (1960).
McClelland, R. A. & Santry, L. J. Reactivity of tetrahedral intermediates. Acc. Chem. Res. 16, 394–399 (1983).
Raynal, M., Ballester, P., Vidal-Ferran, A. & van Leeuwen, P. W. N. M. Supramolecular catalysis. Part 1: non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 43, 1660–1733 (2014).
Steinman, G. & Cole, M. N. Synthesis of biologically pertinent peptides under possible primordial conditions. Proc. Natl Acad. Sci. USA 58, 735–742 (1967).
Toparlak, D., Karki, M., Egas Ortuno, V., Krishnamurthy, R. & Mansy, S. S. Cyclophospholipids increase protocellular stability to metal ions. Small 16, 1903381 (2019).
Rao, M., Eichberg, J. & Oró, J. Synthesis of phosphatidylcholine under possible primitive Earth conditions. J. Mol. Evol. 18, 196–202 (1982).
Frisch, M. J. et al. Gaussian 09, Revision A.02 (Gaussian Inc, 2016).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Delory, D. E. & King, E. J. A sodium carbonate-bicarbonate buffer for alkaline phosphatases. Biochem. J. 39, 245 (1945).
Kempe, S. & Degens, E. T. An early soda ocean? Chem. Geol. 53, 95–108 (1985).
Martin, W. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. B 362, 1887–1925 (2007).
Perkins, W. R. et al. Role of lipid polymorphism in pulmonary surfactant. Science 273, 330–332 (1996).
Athenstaedt, K. & Daum, G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 266, 1–16 (1999).
Chen, I. A. & Szostak, J. W. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc. Natl Acad. Sci. USA 101, 7965–7970 (2004).
Morese, J. W. & Mackenzie, F. T. Hadean ocean carbonate geochemistry. Aquat. Geochem. 4, 301–319 (1998).
Macleod, G., McKeown, C., Hall, A. J. & Russell, M. J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biospheres 24, 19–41 (1994).
Acknowledgements
N.K.D. acknowledges financial support for this work provided by the National Science Foundation (grant no. CHE-1254611) and the National Institutes of Health (grant no. DP2DK111801). K.N.H. acknowledges National Science Foundation (grant no. CHE-1764328), the National Institutes of Health, National Institute of General Medical Sciences (grant no. R01 GM109078), for financial support of this research. S.Q.L. acknowledges financial support for this work provided by National Science Foundation (grant no. OCE-1536702). Computation time was provided by the UCLA Institute for Digital Research and Education (IDRE).
Author information
Authors and Affiliations
Contributions
L.L. and N.K.D. conceived the project. L.L. designed and performed the synthetic experiments. L.L., A.B. and D.Z. performed microscopy experiments. Y.Z. and K.N.H. performed the theoretical study. L.L., Y.Z., A.B., D.Z., S.Q.L., K.N.H. and N.K.D. analysed the data. L.L., Y.Z. and N.K.D. wrote the manuscript. S.Q.L. and K.N.H. assisted in writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and Methods, Supplementary Text, Figs. 1–27, Table 1, copies of HPLC and NMR Spectra.
Rights and permissions
About this article
Cite this article
Liu, L., Zou, Y., Bhattacharya, A. et al. Enzyme-free synthesis of natural phospholipids in water. Nat. Chem. 12, 1029–1034 (2020). https://doi.org/10.1038/s41557-020-00559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00559-0
This article is cited by
-
Periodic temperature changes drive the proliferation of self-replicating RNAs in vesicle populations
Nature Communications (2023)
-
Synthesising a minimal cell with artificial metabolic pathways
Communications Chemistry (2023)
-
Elucidating N-acyl amino acids as a model protoamphiphilic system
Communications Chemistry (2022)
-
Self-reproducing catalytic micelles as nanoscopic protocell precursors
Nature Reviews Chemistry (2021)
-
Synthesis of lipid membranes for artificial cells
Nature Reviews Chemistry (2021)