Abstract
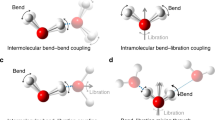
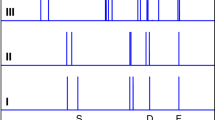
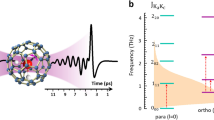
The extremely broad infrared spectrum of water in the OH stretching region is a manifestation of how profoundly a water molecule is distorted when embedded in its extended hydrogen-bonding network. Many effects contribute to this breadth in solution at room temperature, which raises the question as to what the spectrum of a single OH oscillator would be in the absence of thermal fluctuations and coupling to nearby OH groups. We report the intrinsic spectral responses of isolated OH oscillators embedded in two cold (~20 K), hydrogen-bonded water cages adopted by the Cs+·(HDO)(D2O)19 and D3O+·(HDO)(D2O)19 clusters. Most OH oscillators yield single, isolated features that occur with linewidths that increase approximately linearly with their redshifts. Oscillators near 3,400 cm−1, however, occur with a second feature, which indicates that OH stretch excitation of these molecules drives low-frequency, phonon-type motions of the cage. The excited state lifetimes inferred from the broadening are considered in the context of fluctuations in the local electric fields that are available even at low temperature.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary information files.
References
Paesani, F., Xantheas, S. S. & Voth, G. A. Infrared spectroscopy and hydrogen-bond dynamics of liquid water from centroid molecular dynamics with an ab initio-based force field. J. Phys. Chem. B 113, 13118–13130 (2009).
Reddy, S. K., Moberg, D. R., Straight, S. C. & Paesani, F. Temperature-dependent vibrational spectra and structure of liquid water from classical and quantum simulations with the MB-pol potential energy function. J. Chem. Phys. 147, 244504 (2017).
Auer, B. M. & Skinner, J. L. IR and Raman spectra of liquid water: theory and interpretation. J. Chem. Phys. 128, 224511 (2008).
Skinner, J. L., Pieniazek, P. A. & Gruenbaum, S. M. Vibrational spectroscopy of water at interfaces. Acc. Chem. Res. 45, 93–100 (2012).
Ni, Y. C., Gruenbaum, S. M. & Skinner, J. L. Slow hydrogen-bond switching dynamics at the water surface revealed by theoretical two-dimensional sum-frequency spectroscopy. Proc. Natl Acad. Sci. USA 110, 1992–1998 (2013).
Nicodemus, R. A., Corcelli, S. A., Skinner, J. L. & Tokmakoff, A. Collective hydrogen bond reorganization in water studied with temperature-dependent ultrafast infrared spectroscopy. J. Phys. Chem. B 115, 5604–5616 (2011).
Laage, D., Stirnemann, G., Sterpone, F. & Hynes, J. T. Water jump reorientation: from theoretical prediction to experimental observation. Acc. Chem. Res. 45, 53–62 (2012).
Ashihara, S., Huse, N., Espagne, A., Nibbering, E. T. J. & Elsaesser, T. Ultrafast structural dynamics of water induced by dissipation of vibrational energy. J. Phys. Chem. A 111, 743–746 (2007).
Steinel, T. et al. Water dynamics: dependence on local structure probed with vibrational echo correlation spectroscopy. Chem. Phys. Lett. 386, 295–300 (2004).
De Marco, L., Fournier, J. A., Thamer, M., Carpenter, W. & Tokmakoff, A. Anharmonic exciton dynamics and energy dissipation in liquid water from two-dimensional infrared spectroscopy. J. Chem. Phys. 145, 094501 (2016).
De Marco, L., Ramasesha, K. & Tokmakoff, A. Experimental evidence of Fermi resonances in isotopically dilute water from ultrafast broadband IR spectroscopy. J. Phys. Chem. B 117, 15319–15327 (2013).
Asbury, J. B. et al. Dynamics of water probed with vibrational echo correlation spectroscopy. J. Chem. Phys. 121, 12431–12446 (2004).
Stiopkin, I. V. et al. Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy. Nature 474, 192–195 (2011).
Schaefer, J., Backus, E. H. G., Nagata, Y. & Bonn, M. Both inter- and intramolecular coupling of O–H groups determine the vibrational response of the water/air interface. J. Phys. Chem. Lett. 7, 4591–4595 (2016).
van der Post, S. T. et al. Strong frequency dependence of vibrational relaxation in bulk and surface water reveals sub-picosecond structural heterogeneity. Nat. Commun. 6, 8384 (2015).
van der Post, S. T. & Bakker, H. J. Femtosecond mid-infrared study of the reorientation of weakly hydrogen-bonded water molecules. J. Phys. Chem. B 118, 8179–8189 (2014).
Bakker, H. J. & Skinner, J. L. Vibrational spectroscopy as a probe of structure and dynamics in liquid water. Chem. Rev. 110, 1498–1517 (2010).
Skinner, J. L., Auer, B. M. & Lin, Y. S. Vibrational line shapes, spectral diffusion, and hydrogen bonding in liquid water. Adv. Chem. Phys. 142, 59–103 (2009).
Tainter, C. J., Ni, Y., Shi, L. & Skinner, J. L. Hydrogen bonding and OH-stretch spectroscopy in water: hexamer (cage), liquid surface, liquid, and ice. J. Phys. Chem. Lett. 4, 12–17 (2013).
Mallik, B. S., Semparithi, A. & Chandra, A. Vibrational spectral diffusion and hydrogen bond dynamics in heavy water from first principles. J. Phys. Chem. A 112, 5104–5112 (2008).
Stenger, J., Madsen, D., Hamm, P., Nibbering, E. T. J. & Elsaesser, T. Ultrafast vibrational dephasing of liquid water. Phys. Rev. Lett. 87, 027401 (2001).
Asbury, J. B. et al. Ultrafast heterodyne detected infrared multidimensional vibrational stimulated echo studies of hydrogen bond dynamics. Chem. Phys. Lett. 374, 362–371 (2003).
Yang, N., Duong, C. H., Kelleher, P. J., Johnson, M. A. & McCoy, A. B. Isolation of site-specific anharmonicities of individual water molecules in the I-·(H2O)2 complex using tag-free, isotopomer selective IR-IR double resonance. Chem. Phys. Lett. 690, 159–171 (2017).
Horvath, S. et al. Anharmonicities and isotopic effects in the vibrational spectra of X−H2O, ·HDO, and ·D2O [X = Cl, Br, and I] binary complexes. J. Phys. Chem. A 114, 1556–1568 (2010).
Duong, C. H. et al. Disentangling the complex vibrational spectrum of the protonated water trimer, H+(H2O)3, with two-color IR–IR photodissociation of the bare ion and anharmonic VSCF/VCI theory. J. Phys. Chem. Lett. 8, 3782–3789 (2017).
Lawrence, C. P. & Skinner, J. L. Vibrational spectroscopy of HOD in liquid D2O. VII. Temperature and frequency dependence of the OH stretch lifetime. J. Chem. Phys. 119, 3840–3848 (2003).
Gale, G. M., Gallot, G. & Lascoux, N. Frequency-dependent vibrational population relaxation time of the OH stretching mode in liquid water. Chem. Phys. Lett. 311, 123–125 (1999).
Moilanen, D. E. et al. Water inertial reorientation: hydrogen bond strength and the angular potential. Proc. Natl Acad. Sci. USA 105, 5295–5300 (2008).
Imoto, S., Xantheas, S. S. & Saito, S. Ultrafast dynamics of liquid water: energy relaxation and transfer processes of the OH stretch and the HOH bend. J. Phys. Chem. B 119, 11068–11078 (2015).
Schulz, F. & Hartke, B. Dodecahedral clathrate structures and magic numbers in alkali cation microhydration clusters. Chem. Phys. Chem. 3, 98–106 (2002).
Xantheas, S. S. Low-lying energy isomers and global minima of aqueous nanoclusters: structures and spectroscopic features of the pentagonal dodecahedron (H2O)20 and H3O+(H2O)20. Can. J. Chem. Eng. 90, 843–851 (2012).
Buch, V. et al. The single-crystal, basal face of ice I h investigated with sum frequency generation. J. Chem. Phys. 127, 214502 (2007).
Smit, W. J. et al. Excess hydrogen bond at the ice–vapor interface around 200 K. Phys. Rev. Lett. 119, 133003 (2017).
Yang, N., Duong, C. H., Kelleher, P. J., McCoy, A. B. & Johnson, M. A. Deconstructing water’s diffuse OH stretching vibrational spectrum with cold clusters. Science 364, 275–278 (2019).
Bailey, C. G., Kim, J., Dessent, C. E. H. & Johnson, M. A. Vibrational predissociation spectra of I-·(H2O): isotopic labels and weakly bound complexes with Ar and N2. Chem. Phys. Lett. 269, 122–127 (1997).
Leavitt, C. M. et al. Characterizing the intramolecular H− bond and secondary structure in methylated GlyGlyH+ with H2 predissociation spectroscopy. J. Am. Soc. Mass Spectr. 22, 1941–1952 (2011).
Okumura, M., Yeh, L. I., Myers, J. D. & Lee, Y. T. Infrared-spectra of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H3O+·(H2O)n·(H2)m. J. Phys. Chem. 94, 3416–3427 (1990).
Yang, N., Duong, C. H., Kelleher, P. J. & Johnson, M. A. Unmasking rare, large-amplitude motions in D2-tagged I-·(H2O)2 isotopomers with two-color, infrared–infrared vibrational predissociation spectroscopy. J. Phys. Chem. Lett. 9, 3744–3750 (2018).
Wolke, C. T. et al. Isotopomer-selective spectra of a single intact H2O molecule in the Cs+(D2O)5H2O isotopologue: going beyond pattern recognition to harvest the structural information encoded in vibrational spectra. J. Chem. Phys. 144, 074305 (2016).
Fournier, J. A. et al. Site-specific vibrational spectral signatures of water molecules in the magic H3O+(H2O)20 and Cs+(H2O)20 clusters. Proc. Natl Acad. Sci. USA 111, 18132–18137 (2014).
Fournier, J. A. et al. Vibrational spectral signature of the proton defect in the three-dimensional H+(H2O)21 cluster. Science 344, 1009–1012 (2014).
Fournier, J. A. et al. Snapshots of proton accommodation at a microscopic water surface: understanding the vibrational spectral signatures of the charge defect in cryogenically cooled H+(H2O)(n=2–28) clusters. J. Phys. Chem. A 119, 9425–9440 (2015).
Johnson, C. J. et al. Microhydration of contact ion pairs in M2+OH–(H2O)n=1–5 (M = Mg, Ca) clusters: spectral manifestations of a mobile proton defect in the first hydration shell. J. Phys. Chem. A 118, 7590–7597 (2014).
Hamm, P. & Stock, G. Nonadiabatic vibrational dynamics in the HCO2 –·H2O complex. J. Chem. Phys 143, 134308 (2015).
Acknowledgements
We acknowledge extensive discussions with A. B. McCoy on the possible origins of the broadening, as well as unpublished results shared by S. Xantheas on variations in the low-energy structures available to the Cs+·(H2O)20 clusters. This work was supported by the National Science Foundation grant CHE-1900119 and carried out with the instrument developed under AFOSR DURIP grant FA9550-17-1-0267. The part of the study on the H3O+(H2O)20 cluster was carried out under US Department of Energy, Office of Science, Basic Energy Sciences, CPIMS Program under grant award DE-FG02-06ER15800 as part of a larger effort designed to determine the barriers for proton translocation through the cage structure. C.H.D. thanks the National Science Foundation Graduate Research Fellowship for funding under grant DGE‐1122492.
Author information
Authors and Affiliations
Contributions
M.A.J. conceptualized and supervised the experiments, N.Y., C.H.D. and P.J.K. performed the experiments, N.Y. analysed the data, performed calculations and wrote the simulation codes. All the authors discussed the results and worked on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–8, Tables 1–3 and calculations.
Supplementary Data 1
Raw data used to generate the figures.
Cs 57 mode
Representative low-energy vibrational mode of Cs+·(H2O)20 cluster (57 cm−1).
Cs 60 mode
Representative low-energy vibrational mode of Cs+·(H2O)20 cluster (60 cm−1).
Cs 63 mode
Representative low-energy vibrational mode of Cs+·(H2O)20 cluster (63 cm−1).
Cs 65 mode
Representative low-energy vibrational mode of Cs+·(H2O)20 cluster (65 cm−1).
H 60 mode
Representative low-energy vibrational mode of H3O+·(H2O)20 cluster (60 cm−1).
H 61 mode
Representative low-energy vibrational mode of H3O+·(H2O)20 cluster (61 cm−1).
H 63 mode
Representative low-energy vibrational mode of H3O+·(H2O)20 cluster (63 cm−1).
H 64 mode
Representative low-energy vibrational mode of H3O+·(H2O)20 cluster (64 cm-1).
Rights and permissions
About this article
Cite this article
Yang, N., Duong, C.H., Kelleher, P.J. et al. Capturing intrinsic site-dependent spectral signatures and lifetimes of isolated OH oscillators in extended water networks. Nat. Chem. 12, 159–164 (2020). https://doi.org/10.1038/s41557-019-0376-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0376-9
This article is cited by
-
Towards complete assignment of the infrared spectrum of the protonated water cluster H+(H2O)21
Nature Communications (2021)