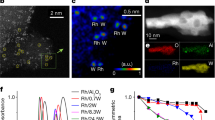
Abstract
In an effort to obtain the maximum atom efficiency, research on heterogeneous single-atom catalysts has intensified recently. Anchoring organometallic homogeneous catalysts to surfaces creates issues with retaining mononuclearity and activity, while the several techniques developed to prepare atomically dispersed precious metals on oxide supports are usually complex. Here we report a facile one-pot synthesis of inorganometallic mononuclear gold complexes formed in alkaline solutions as robust and versatile single-atom gold catalysts. The complexes remain intact on impregnation onto supports or after drying in air to give a crystalline powder. They can be used to interrogate the nuclearity of the catalytically active gold site for reactions known to be catalysed by oxidized gold species. We show that the [Au1–Ox]– cluster directs the heterogeneous coupling of two methanol molecules to methyl formate and hydrogen with a 100% selectivity below 180 °C. The reaction is industrially important as well as the key step in methanol steam reforming on gold catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Additional methods and data are provided in the Supplementary Information. All data supporting the findings of this study are available upon request from the corresponding authors.
References
Flytzani-Stephanopoulos, M. & Gates, B. C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 3, 545–574 (2012).
Thomas, J. M. The concept, reality and utility of single-site heterogeneous catalysts (SSHCs). Phys. Chem. Chem. Phys. 16, 7647–7661 (2014).
Oliver-Meseguer, J., Cabrero-Antonino, J. R., Domínguez, I., Leyva-Pérez, A. & Corma, A. Small gold clusters formed in solution give reaction turnover numbers of 107 at room temperature. Science 338, 1452–1455 (2012).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water–gas shift catalysts. Science 301, 935–938 (2003).
Fu, Q., Deng, W., Saltsburg, H. & Flytzani-Stephanopoulos, M. Activity and stability of low-content gold–cerium oxide catalysts for the water–gas shift reaction. Appl. Catal. B 56, 57–68 (2005).
Si, R. & Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au–CeO2 catalysts for the water–gas shift reaction. Angew. Chem. Int. Ed. 47, 2884–2887 (2008).
Hatanaka, M. et al. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by a Pt–O–Ce bond. Appl. Catal. B 99, 336–342 (2010).
Allard, L. F. et al. Evolution of gold structure during thermal treatment of Au/FeOx catalysts revealed by aberration-corrected electron microscopy. J. Electron. Microsc. 58, 199–212 (2009).
Yang, M., Allard, L. F. & Flytzani-Stephanopoulos, M. Atomically dispersed Au–(OH)x species bound on titania catalyze the low-temperature water–gas shift reaction. J. Am. Chem. Soc. 135, 3768–3771 (2013).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Zhao, Y. et al. Stable iridium dinuclear heterogeneous catalysts supported on metal–oxide substrate for solar water oxidation. Proc. Natl Acad. Sci. USA 115, 2902–2907 (2018).
Zhai, Y. et al. Alkali-stabilized Pt–OHx species catalyze low-temperature water–gas shift reactions. Science 329, 1633–1636 (2010).
Yang, M. et al. Catalytically active Au–O(OH)x-species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Yang, M. et al. A common single-site Pt(ii)–O(OH)x- species stabilized by sodium on ‘active’ and ‘inert’ supports catalyzes the water–gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Johnston, H. L. & Leland, H. L. The solubility of gold hydroxide in alkali and equilibria in the saturated solutions. J. Am. Chem. Soc. 60, 1439–1445 (1938).
Bircumshaw, L. L. The formation of gold sols in alkaline solutions. J. Chem. Soc. Faraday Trans. 34, 1236–1238 (1938).
Lee, S., Fan, C., Wu, T. & Anderson, S. L. Agglomeration, support effects, and CO adsorption on Au/TiO2(110) prepared by ion beam deposition. Surf. Sci. 578, 5–19 (2005).
Deng, W., Carpenter, C., Yi, N. & Flytzani-Stephanopoulos, M. Comparison of the activity of Au/CeO2 and Au/Fe2O3 catalysts for the CO oxidation and the water–gas shift reactions. Top. Catal. 44, 199–208 (2007).
Adrian, R., Fernandez-Cestau, J., Morris, J., Wright, J. A. & Bochmann, M. Gold(iii)–CO and gold(iii)–CO2 complexes and their role in the water–gas shift reaction. Sci. Adv. 1, e1500761 (2015).
Liu, Z., Jenkins, S. J. & King, D. A. Origin and activity of oxidized gold in water–gas-shift catalysis. Phys. Rev. Lett. 94, 196102 (2005).
Palo, D. R., Dagle, R. A. & Holladay, J. D. Methanol steam reforming for hydrogen production. Chem. Rev. 107, 3992–4021 (2007).
Yi, N., Si, R., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active gold species on cerium oxide nanoshapes for methanol steam reforming and the water gas shift reactions. Energy Environ. Sci. 3, 831–837 (2010).
Boucher, M. B. et al. Hydrogen production from methanol over gold supported on ZnO and CeO2 nanoshapes. J. Phys. Chem. C 115, 1261–1268 (2011).
Enache, D. I. et al. Solvent-free oxidation of primary alcohols to aldehydes using Au–Pd/TiO2 catalysts. Science 311, 362–365 (2006).
Wittstock, A., Zielasek, V., Biener, J., Friend, C. M. & Baumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327, 319–322 (2010).
Xu, B., Liu, X., Haubrich, J., Madix, R. J. & Friend, C. M. Selectivity control in gold-mediated esterification of methanol. Angew. Chem. Int. Ed. 48, 4206–4209 (2009).
Stowers, K. J., Madix, R. J. & Friend, C. M. From model studies on Au(111) to working conditions with unsupported nanoporous gold catalysts: oxygen-assisted coupling reactions. J. Catal. 308, 131–141 (2013).
Zugic, B. et al. Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts. Nat. Mater. 16, 558–564 (2017).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Malta, G. et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355, 1399–1403 (2017).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80 (2017).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Whiting, G. T. et al. Methyl formate formation from methanol oxidation using supported gold–palladium nanoparticles. ACS Catal. 5, 637–644 (2015).
Busca, G., Elmi, A. S. & Forzatti, P. Mechanism of selective methanol oxidation over vanadium oxide–titanium oxide catalysts: a FTIR and flow reactor study. J. Phys. Chem. 91, 5263–5269 (1987).
Larrubia Vargas, M. A. et al. An IR study of methanol steam reforming over ex-hydrotalcite Cu–Zn–Al catalysts. J. Mol. Catal. A 266, 188–197 (2007).
Wojcieszak, R., Karelovic, A., Gaigneaux, E. M. & Ruiz, P. Oxidation of methanol to methyl formate over supported Pd nanoparticles: insights into the reaction mechanism at low temperature. Catal. Sci. Technol. 4, 3298–3305 (2014).
Lochař, V. FT-IR study of methanol, formaldehyde and methyl formate adsorption on the surface of Mo/Sn oxide catalyst. Appl. Catal. A 309, 33–36 (2006).
Acknowledgements
The financial support by the DOE/BES under Grant no. DE-FG02-05ER15730 is acknowledged. The XAS research is sponsored by the Advanced Photon Source at Argonne National Laboratory under Contract no. DE-AC02-06CH11357. The aberration-corrected microscopy research conducted at Oak Ridge National Laboratory was sponsored by the US DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office, Propulsion Materials Program. This work was also supported by DOE-BES, Office of Chemical Sciences (Grant DE-FG02-05ER15731). Calculations were performed at supercomputing centres located at the Environmental Molecular Sciences Laboratory, which is sponsored by the DOE Office of Biological and Environmental Research at the Pacific Northwest National Laboratory, the Center for Nanoscale Materials at Argonne National Laboratory, supported by DOE contract DE-AC02-06CH11357, the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility supported by DOE contract DE-AC02-05CH11231 and the UW-Madison Center for High Throughput Computing (CHTC), supported by UW-Madison, the Advanced Computing Initiative, the Wisconsin Alumni Research Foundation, the Wisconsin Institutes for Discovery, and the National Science Foundation, and is an active member of the Open Science Grid, which is supported by the National Science Foundation and the US Department of Energy’s Office of Science.
Author information
Authors and Affiliations
Contributions
M.Y. developed the sample synthesis approach with S.C.; S.C., M.Y. and C.W. performed the catalytic tests for methanol self-coupling, WGS and methanol steam reforming reactions. A.O.E., F.G. and S.L. conducted the DFT calculations. S.C. and A.T. conducted the infrared study. S.C., M.Y, S.L., J.S. and M.L. performed the XAS experiments. K.W.C. and Z.C. performed the pair distribution function experiments. L.F.A. performed the microscopy work, S.C. and J.L. conducted the XRD, and T.H. analysed the XRD data. S.C., M.Y., A.O.E., M.M. and M.F.-S. wrote the manuscript. M.M. and M.F.-S. guided the work and coordinated the individual author contributions. All the authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary experimental methods, X-ray characterization (XRD, XPS and XAS), DFT methods and results, Figs. 1–50 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Cao, S., Yang, M., Elnabawy, A.O. et al. Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions. Nat. Chem. 11, 1098–1105 (2019). https://doi.org/10.1038/s41557-019-0345-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0345-3
This article is cited by
-
Co-catalytic metal–support interactions in single-atom electrocatalysts
Nature Reviews Materials (2024)
-
Strategies to improve hydrogen activation on gold catalysts
Nature Reviews Chemistry (2024)
-
Methanol Oxidation Catalytic Performance Enhancement via Constructing Pd-MgAl2O4 Interface and its Reaction Mechanism Investigation
Catalysis Letters (2023)
-
Research progress on electrochemical CO2 reduction for Cu-based single-atom catalysts
Science China Materials (2023)
-
Solid catalysts for the dehydrogenation of long-chain alkanes: lessons from the dehydrogenation of light alkanes and homogeneous molecular catalysis
Science China Chemistry (2022)