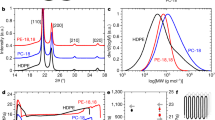
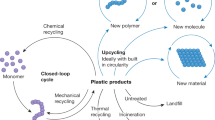
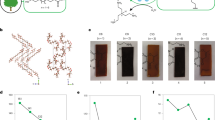
Abstract
Recycled plastics are low-value commodities due to residual impurities and the degradation of polymer properties with each cycle of re-use. Plastics that undergo reversible polymerization allow high-value monomers to be recovered and re-manufactured into pristine materials, which should incentivize recycling in closed-loop life cycles. However, monomer recovery is often costly, incompatible with complex mixtures and energy-intensive. Here, we show that next-generation plastics—polymerized using dynamic covalent diketoenamine bonds—allow the recovery of monomers from common additives, even in mixed waste streams. Poly(diketoenamine)s ‘click’ together from a wide variety of triketones and aromatic or aliphatic amines, yielding only water as a by-product. Recovered monomers can be re-manufactured into the same polymer formulation, without loss of performance, as well as other polymer formulations with differentiated properties. The ease with which poly(diketoenamine)s can be manufactured, used, recycled and re-used—without losing value—points to new directions in designing sustainable polymers with minimal environmental impact.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information, and also from the authors upon request. Crystallographic data for compounds 3, 5 and TK-6 are available free of charge from the Cambridge Crystallographic Date Centre (www.ccdc.cam.ac.uk) under reference nos. 1891131, 1891132 and 189113, respectively.
References
Helms, B. A. & Russell, T. P. Polymer chemistries enabling cradle-to-cradle life cycles for plastics. Chem. 1, 813–819 (2016).
Rahimi, A. R. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
García, J. M. & Robertson, M. L. The future of plastics recycling. Science 358, 870–872 (2017).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706 (2017).
MacArthur, E. Beyond plastic waste. Science 358, 843 (2017).
Schneiderman, D. K. & Hillmyer, M. A. 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules 50, 3733–3749 (2017).
Sardon, H. & Dove, A. P. Plastics recycling with a difference. Science 360, 380–381 (2018).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Zhang, X., Fevre, M., Jones, G. O. & Waymouth, R. M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 118, 839–885 (2018).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Paszun, D. & Spychaj, T. Chemical recycling of poly(ethylene terephthalate). Ind. Eng. Chem. Res. 36, 1373–1383 (1997).
Yoshioka, T., Motoki, T. & Okuwaki, A. Kinetics of hydrolysis of poly(ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Chem. Res. 40, 75–79 (2001).
Kamber, N. E. et al. The depolymerization of poly(ethylene terephthalate) (PET) using N-heterocyclic carbenes from ionic liquids. J. Chem. Educ. 87, 519–521 (2010).
Fukushima, K. et al. Organocatalytic depolymerization of poly(ethylene terephthalate). J. Polym. Sci. A 49, 1273–1281 (2011).
Fukushima, K. et al. Advanced chemical recycling of poly(ethylene terephthalate) through organocatalytic aminolysis. Polym. Chem. 4, 1610–1616 (2013).
Ying, H., Zhang, Y. & Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 5, 3218 (2014).
Zhang, Y. et al. Malleable and recyclable poly(urea-urethane) thermosets bearing hindered urea bonds. Adv. Mater. 28, 7646–7651 (2016).
Jia, X., Qin, C., Friedberger, T., Guan, Z. & Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2, e1501591 (2016).
Jones, G. O., Yuen, A., Wojtecki, R. J., Hedrick, J. L. & García, J. M. Computational and experimental investigations of one-step conversion of poly(carbonate)s into value-added poly(aryl ether sulfone)s. Proc. Natl Acad. Sci. USA 113, 7722–7726 (2016).
García, J. M. et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science 344, 732–735 (2014).
Schneiderman, D. K. et al. Chemically recyclable biobased polyurethanes. ACS Macro. Lett. 5, 515–518 (2016).
MacDonald, J. P. & Shaver, M. P. An aromatic/aliphatic polyester prepared via ring-opening polymerization and its remarkable selective and cyclable depolymerization to monomer. Polym. Chem. 7, 553–559 (2016).
Tang, X. et al. The quest for converting biorenewable bifunctional α-methylene-γ-butyrolactone into degradable and recyclable polyester: controlling vinyl-addition/ring-opening/cross-linking pathways. J. Am. Chem. Soc. 138, 14326–14337 (2016).
Hong, M. & Chen, E. Y.-X. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained γ-butyrolactone. Angew. Chem. Int. Ed. 55, 4188–4193 (2016).
Hong, M. & Chen, E. Y.-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 8, 42–49 (2016).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Montarnal, D., Capelot, M., Tournilhac, F. & Leibler, L. Silica-like malleable materials from permanent organic networks. Science 334, 965–968 (2011).
Denissen, W. et al. Vinylogous urethane vitrimers. Adv. Funct. Mater. 25, 2451–2457 (2015).
Fortman, D. J., Brutman, J. P., Cramer, C. J., Hillmyer, M. A. & Dichtel, W. R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 137, 14019–14022 (2015).
Obadia, M. M., Mudraboyina, B. P., Serghei, A., Montarnal, D. & Drockenmuller, E. Reprocessing and recycling of highly cross-linked ion-conduction networks through trans-alkylation exchanges of C–N bonds. J. Am. Chem. Soc. 137, 6078–6083 (2015).
Taynton, P. et al. Repairable woven carbon-fiber composites with full recyclability enabled by malleable polyimine networks. Adv. Mater. 28, 2904–2909 (2016).
Yu, K., Shi, Q., Dunn, M. L., Wang, T. & Qi, H. J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 26, 6098–6106 (2016).
Rötger, M. et al. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 356, 62–65 (2017).
Snyder, R. L., Fortman, D. J., De Hoe, G. X., Hillmyer, M. A. & Dichtel, W. R. Reprocessable acid-degradable polycarbonate vitrimers. Macromolecules 51, 389–397 (2018).
Zou, Z. et al. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 4, eaaq0508 (2018).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Kohout, M., Bielec, B., Steindl, P., Trettenhahn, G. & Lindner, W. Mechanistic aspects of the direct C-acylation of cyclic 1,3-diones with various unactivated carboxylic acids. Tetrahedron 71, 2698–2707 (2015).
Augustyns, K., Kraas, W. & Jung, G. Investigation on the stability of the Dde protecting group used in peptide synthesis: migration to an unprotected lysine. J. Peptide Res. 51, 127–133 (1998).
Chong, J. H., Sauer, M., Patrick, B. O. & MacLachlan, M. J. Highly stable keto-enamine salicylideneanilines. Org. Lett. 5, 3823–3826 (2003).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012).
DeBlase, C. R., Silberstein, K. E., Truong, T.-T., Abruña, H. D. & Dichtel, W. R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135, 16821–16824 (2013).
Taylor, M. $180bn investment in plastic factories feeds global packaging binge. The Guardian (26 December 2017); https://www.theguardian.com/environment/2017/dec/26/180bn-investment-in-plastic-factories-feeds-global-packaging-binge
Acknowledgements
The technical scope of this work was supported by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory under US Department of Energy contract no. DE-AC02–05CH11231. K.E.L. was supported by the US Department of Energy, Office of Science, Office of Workforce Development for Teachers and Scientists (WDTS) under the Science Undergraduate Laboratory Internship (SULI) programme. Portions of this work, including organic and polymer synthesis and characterization, were carried out as a User Project at the Molecular Foundry, which is supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
B.A.H. and P.R.C. designed and planned the project. P.R.C. synthesized and characterized all PDK materials and their recyclability. A.M.S. synthesized small molecules and carried out experiments to measure the activation energies for amine exchange. K.E.L. carried out experiments to characterize the extent of network formation by ball-milling. B.A.H. and P.R.C. wrote the manuscript, with contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
B.A.H. and P.R.C. are inventors on US provisional patent application 62/587,148 submitted by Lawrence Berkeley National Laboratory that covers poly(diketoenamine)s, as well as aspects of their use and recovery.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary synthetic procedures, materials characterization, closed-loop polymer recycling, bond exchange kinetics, and Supplementary Figures 1–17.
Crystallographic data
CIF for compound 3; CCDC reference: 1891131.
Crystallographic data
CIF for compound 5; CCDC reference: 1891132.
Crystallographic data
CIF for compound TK-6; CCDC reference: 1891133.
Rights and permissions
About this article
Cite this article
Christensen, P.R., Scheuermann, A.M., Loeffler, K.E. et al. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 11, 442–448 (2019). https://doi.org/10.1038/s41557-019-0249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0249-2
This article is cited by
-
Inhibition of iRhom1 by CD44-targeting nanocarrier for improved cancer immunochemotherapy
Nature Communications (2024)
-
Molecular design of recyclable thermosetting polyimide and its composite with excellent mechanical and tribological properties
Friction (2024)
-
Biobased, biodegradable and compostable plastics: chemical nature, biodegradation pathways and environmental strategy
Environmental Science and Pollution Research (2024)
-
Design of covalent adaptable networks with intrinsic flame retardancy
Polymer Bulletin (2024)
-
End-of-life upcycling of polyurethanes using a room temperature, mechanism-based degradation
Nature Chemistry (2023)