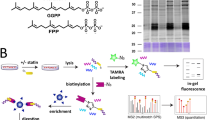
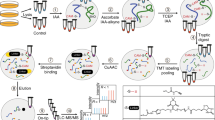
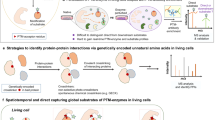
Abstract
Post-translational farnesylation or geranylgeranylation at a C-terminal cysteine residue regulates the localization and function of over 100 proteins, including the Ras isoforms, and is a therapeutic target in diseases including cancer and infection. Here, we report global and selective profiling of prenylated proteins in living cells enabled by the development of isoprenoid analogues YnF and YnGG in combination with quantitative chemical proteomics. Eighty prenylated proteins were identified in a single human cell line, 64 for the first time at endogenous abundance without metabolic perturbation. We further demonstrate that YnF and YnGG enable direct identification of post-translationally processed prenylated peptides, proteome-wide quantitative analysis of prenylation dynamics and alternative prenylation in response to four different prenyltransferase inhibitors, and quantification of defective Rab prenylation in a model of the retinal degenerative disease choroideremia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are available from the authors. The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository49, with data set identifier PXD009155.
References
Wang, M. & Casey, P. J. Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 17, 110–122 (2016).
Berndt, N., Hamilton, A. D. & Sebti, S. M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11, 775–791 (2011).
Oesterle, A., Laufs, U. & Liao, J. K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243 (2017).
Roosing, S., Collin, R. W., den Hollander, A. I., Cremers, F. P. & Siemiatkowska, A. M. Prenylation defects in inherited retinal diseases. J. Med. Genet. 51, 143–151 (2014).
Wang, C. et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol. Cell 18, 425–434 (2005).
Koh, C. et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect. Dis. 15, 1167–1174 (2015).
Gordon, L. B. et al. Impact of farnesylation inhibitors on survival in Hutchinson–Gilford progeria syndrome. Circulation 130, 27–34 (2014).
Grammel, M. & Hang, H. C. Chemical reporters for biological discovery. Nat. Chem. Biol. 9, 475–484 (2013).
Tate, E. W., Kalesh, K. A., Lanyon-Hogg, T., Storck, E. M. & Thinon, E. Global profiling of protein lipidation using chemical proteomic technologies. Curr. Opin. Chem. Biol. 24, 48–57 (2015).
Grocin, A. G., Serwa, R. A., Sanfrutos, J. M., Ritzefeld, M. & Tate, E. W. Whole proteome profiling of N-myristoyltransferase activity and inhibition using sortase A. Mol. Cell. Proteomics 18, 115–126 (2019).
Kho, Y. et al. A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc. Natl Acad. Sci. USA 101, 12479–12484 (2004).
Chan, L. N. et al. A novel approach to tag and identify geranylgeranylated proteins. Electrophoresis 30, 3598–3606 (2009).
Nguyen, U. T. et al. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat. Chem. Biol. 5, 227–235 (2009).
Berry, A. F. et al. Rapid multilabel detection of geranylgeranylated proteins by using bioorthogonal ligation chemistry. Chembiochem 11, 771–773 (2010).
Charron, G., Li, M. M., MacDonald, M. R. & Hang, H. C. Prenylome profiling reveals S-farnesylation is crucial for membrane targeting and antiviral activity of ZAP long-isoform. Proc. Natl Acad. Sci. USA 110, 11085–11090 (2013).
Palsuledesai, C. C. et al. Metabolic labeling with an alkyne-modified isoprenoid analog facilitates imaging and quantification of the prenylome in cells. ACS Chem. Biol. 11, 2820–2828 (2016).
Thinon, E. et al. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 5, 4919 (2014).
Broncel, M. et al. Multifunctional reagents for quantitative proteome-wide analysis of protein modification in human cells and dynamic profiling of protein lipidation during vertebrate development. Angew. Chem. Int. Ed. 54, 5948–5951 (2015).
van Oost, B. A., Edgell, C. J., Hay, C. W. & MacGillivray, R. T. Isolation of a human von Willebrand factor cDNA from the hybrid endothelial cell line EA.hy926. Biochem. Cell Biol. 64, 699–705 (1986).
Holstein, S. A., Wohlford-Lenane, C. L. & Hohl, R. J. Consequences of mevalonate depletion. Differential transcriptional, translational and post-translational up-regulation of Ras, Rap1a, RhoA and RhoB. J. Biol. Chem. 277, 10678–10682 (2002).
Geiger, T. et al. Use of stable isotope labeling by amino acids in cell culture as a spike-in standard in quantitative proteomics. Nat. Protoc. 6, 147–157 (2011).
Liu, Z. et al. Membrane-associated farnesylated UCH-L1 promotes α-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc. Natl Acad. Sci. USA 106, 4635–4640 (2009).
Clulow, J. A. et al. Competition-based, quantitative chemical proteomics in breast cancer cells identifies new target profiles for sulforaphane. Chem. Commun. 53, 5182–5185 (2017).
Kalesh, K. A., Clulow, J. A. & Tate, E. W. Target profiling of zerumbone using a novel cell-permeable clickable probe and quantitative chemical proteomics. Chem. Commun. 51, 5497–5500 (2015).
Wright, M. H. et al. Global analysis of protein N-myristoylation and exploration of N-myristoyltransferase as a drug target in the neglected human pathogen Leishmania donovani. Chem. Biol. 22, 342–354 (2015).
Huesgen, P. F. et al. LysargiNase mirrors trypsin for protein C-terminal and methylation-site identification. Nat. Methods 12, 55–58 (2015).
Kassai, H., Satomi, Y., Fukada, Y. & Takao, T. Top-down analysis of protein isoprenylation by electrospray ionization hybrid quadrupole time-of-flight tandem mass spectrometry; the mouse Tgamma protein. Rapid Commun. Mass Spectrom. 19, 269–274 (2005).
Diaz-Rodriguez, V. et al. a-Factor analogues containing alkyne- and azide-functionalized isoprenoids are efficiently enzymatically processed and retain wild type bioactivity. Bioconjug. Chem. 29, 316–323 (2017).
McGuire, T. F., Qian, Y., Vogt, A., Hamilton, A. D. & Sebti, S. M. Platelet-derived growth factor receptor tyrosine phosphorylation requires protein geranylgeranylation but not farnesylation. J. Biol. Chem. 271, 27402–27407 (1996).
Hara, M. et al. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc. Natl Acad. Sci. USA 90, 2281–2285 (1993).
End, D. W. et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 61, 131–137 (2001).
Carboni, J. M. et al. Farnesyltransferase inhibitors are inhibitors of Ras but not R-Ras2/TC21, transformation. Oncogene 10, 1905–1913 (1995).
Coussa, R. G. & Traboulsi, E. I. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 33, 57–65 (2012).
Dimopoulos, I. S., Radziwon, A., St Laurent, C. D. & MacDonald, I. M. Choroideremia. Curr. Opin. Ophthalmol. 28, 410–415 (2017).
Seabra, M. C., Brown, M. S. & Goldstein, J. L. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science 259, 377–381 (1993).
Edwards, T. L. et al. Visual acuity after retinal gene therapy for choroideremia. N. Engl. J. Med. 374, 1996–1998 (2016).
Xue, K. et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 24, 1507–1512 (2018).
Tolmachova, T. et al. Independent degeneration of photoreceptors and retinal pigment epithelium in conditional knockout mouse models of choroideremia. J. Clin. Invest. 116, 386–394 (2006).
Jennings, B. C. et al. Analogs of farnesyl diphosphate alter CaaX substrate specificity and reactions rates of protein farnesyltransferase. Bioorg. Med. Chem. Lett. 26, 1333–1336 (2016).
Liu, Y. et al. Direct binding of CEP85 to STIL ensures robust PLK4 activation and efficient centriole assembly. Nat. Commun. 9, 1731 (2018).
Caballe, A. et al. ULK3 regulates cytokinetic abscission by phosphorylating ESCRT-III proteins. Elife 4, e06547 (2015).
Goruppi, S. et al. The ULK3 Kinase is critical for convergent control of cancer-associated fibroblast activation by CSL and GLI. Cell Rep. 20, 2468–2479 (2017).
Douchi, D. et al. Silencing of LRRFIP1 reverses the epithelial-mesenchymal transition via inhibition of the Wnt/β-catenin signaling pathway. Cancer Lett. 365, 132–140 (2015).
Maurer-Stroh, S. et al. Towards complete sets of farnesylated and geranylgeranylated proteins. PLoS Comput. Biol. 3, e66 (2007).
Onono, F. O. et al. A tagging-via-substrate approach to detect the farnesylated proteome using two-dimensional electrophoresis coupled with western blotting. Mol. Cell. Proteomics 9, 742–751 (2010).
Schilling, O., Barre, O., Huesgen, P. F. & Overall, C. M. Proteome-wide analysis of protein carboxy termini: C terminomics. Nat. Methods 7, 508–511 (2010).
Hildebrandt, E. R. et al. A shunt pathway limits the CaaX processing of Hsp40 Ydj1p and regulates Ydj1p-dependent phenotypes. Elife 5, e15899 (2016).
Blanden, M. J. et al. Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome. J. Biol. Chem. 293, 2770–2785 (2018).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Acknowledgements
The authors thank L. Haigh (Department of Chemistry Mass Spectrometry Facility, Imperial College London) for assistance in acquiring nanoLC–MS/MS and high-resolution mass spectrometry (HRMS) data, S. Sheppard and B. Chappell for their contributions to prenyl probe synthesis, N. O’Reilly (Francis Crick Institute) for peptide substrate synthesis and A.I. Magee (Imperial College London) for insightful comments on the manuscript. This study was supported by Cancer Research UK (Programme Foundation Award C29637/A20183 to E.W.T.), the British Heart Foundation (PhD studentship to E.M.S. and Project Grant PG/12/67/29773 to B.W.S. and E.W.T.), the European Union Framework Programme 7 (Marie Curie Intra European Fellowship to J.Mo.-S. and E.W.T.), Wellcome Trust (Programme Grant 093445/Z/10/Z to M.C.S. and WT102871MA to J.Ma.-S.) and a Royal Thai Government scholarship (PhD studentship to N.P.).
Author information
Authors and Affiliations
Contributions
E.M.S., J.Mo.-S., R.A.S., N.P., B.W.-S. and E.W.T designed the experiments. E.M.S. performed chemical syntheses of reagents, initial method validation and optimization, experiments related to isoprenoid competition and inhibitor treatment, and analysed data. J.Mo.-S. performed experiments related to isoprenoid competition, inhibitor treatment, prenylation dynamics and Rep-1 knockout, and analysed data. R.A.S. performed chemical synthesis of reagents, conducted preliminary experiments and performed proteomic data analysis. N.P. performed chemical synthesis of pyrophosphate derivatives, biochemical enzyme assays, experiments related to concentration-dependent probe incorporation and analysed data. T.L.-H. designed the biochemical enzyme assays and analysed data. T.T. and M.C.S. provided mouse embryonic fibroblasts. L.N.V. and J.Ma.-S. provided ULK3 and CEP85 protein constructs. E.W.T. conceived and directed the study. E.M.S., J.Mo.-S., R.A.S. and E.W.T. wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–29, Supplementary Table 1, and Supplementary Methods.
Supplementary Data 1
Summary of data on 96 prenylated proteins identified in this study, including literature analysis of prior evidence for prenylation.
Supplementary Data 2
Whole proteome analysis of isoprenoid competition versus YnF and YnGG.
Supplementary Data 3
Whole proteome analysis of YnF and YnGG probe concentration gradient
Supplementary Data 4
Whole proteome analysis of prenylated, probe-modified peptides
Supplementary Data 5
Whole proteome analysis of YnF labelling in response to FTI-277, Tipifarnib and Manumycin A
Supplementary Data 6
Whole proteome analysis of YnGG labelling in response to GGTI-2133
Supplementary Data 7
Whole proteome analysis of prenyl probe preference and prenylation switch in response to Tipifarnib
Supplementary Data 8
Whole proteome prenylation analysis in Rep-1 knock-out fibroblasts vs wild type fibroblasts
Rights and permissions
About this article
Cite this article
Storck, E.M., Morales-Sanfrutos, J., Serwa, R.A. et al. Dual chemical probes enable quantitative system-wide analysis of protein prenylation and prenylation dynamics. Nat. Chem. 11, 552–561 (2019). https://doi.org/10.1038/s41557-019-0237-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0237-6
This article is cited by
-
Protein lipidation in cancer: mechanisms, dysregulation and emerging drug targets
Nature Reviews Cancer (2024)
-
Detecting diagnostic features in MS/MS spectra of post-translationally modified peptides
Nature Communications (2023)
-
Farnesyltransferase inhibitor LNK-754 attenuates axonal dystrophy and reduces amyloid pathology in mice
Molecular Neurodegeneration (2022)
-
The emerging role of mass spectrometry-based proteomics in drug discovery
Nature Reviews Drug Discovery (2022)
-
Metabolic labeling with an alkyne probe reveals similarities and differences in the prenylomes of several brain-derived cell lines and primary cells
Scientific Reports (2021)