Abstract
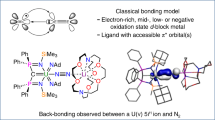
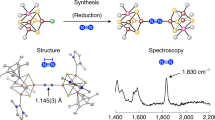
Cooperativity between metal centres is identified as a crucial step in dinitrogen reduction both for the industrial Haber–Bosch process and for the natural fixation of nitrogen by nitrogenase enzymes, but the mechanism of N2 reduction remains poorly understood. This is in large part because multimetallic complexes that reduce and functionalize dinitrogen in the absence of strong alkali reducing agents are crucial to establish a structure–activity relationship, but remain extremely rare. Recently, we reported a multimetallic nitride-bridged diuranium(iii) complex capable of reducing and functionalizing dinitrogen. Here we show that an analogous complex assembled with an oxo instead of a nitride linker also effects the four-electron reduction of dinitrogen, but the reactivity of the resulting oxo–(N2) complex differs significantly from that of the nitride–(N2). Computational studies show a different bonding scheme for the dinitrogen where the bridging nitride does participate in the binding and consequent activation of N2, while the oxide does not.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in this Article and its Supplementary Information or are available from the corresponding author upon reasonable request. Atomic coordinates and structure factors for the reported crystal structures have been deposited in the Cambridge Crystallographic Data Centre under accession codes CCDC 1821630 (2), 1821631 (3), 1821633 (4), 1821634 (5) and 1821632 (6).
References
Burford, R. J. & Fryzuk, M. D. Examining the relationship between coordination mode and reactivity of dinitrogen. Nat. Rev. Chem. 1, 0026 (2017).
Shaver, M. P. & Fryzuk, M. D. Activation of molecular nitrogen: coordination, cleavage and functionalization of N2 mediated by metal complexes. Adv. Synth. Catal. 345, 1061–1076 (2003).
Pool, J. A., Lobkovsky, E. & Chirik, P. J. Hydrogenation and cleavage of dinitrogen to ammonia with a zirconium complex. Nature 427, 527–530 (2004).
Fryzuk, M. D., Love, J. B., Rettig, S. J. & Young, V. G. Transformation of coordinated dinitrogen by reaction with dihydrogen and primary silanes. Science 275, 1445–1447 (1997).
Wang, B. L. et al. Dinitrogen activation by dihydrogen and a PNP-ligated titanium complex. J. Am. Chem. Soc. 139, 1818–1821 (2017).
Keane, A. J., Farrell, W. S., Yonke, B. L., Zavalij, P. Y. & Sita, L. R. Metal-mediated production of isocyanates, R3EN=C=O from dinitrogen, carbon dioxide, and R3ECl. Angew. Chem. Int. Ed. 54, 10220–10224 (2015).
Knobloch, D. J., Lobkovsky, E. & Chirik, P. J. Dinitrogen cleavage and functionalization by carbon monoxide promoted by a hafnium complex. Nat. Chem. 2, 30–35 (2010).
Falcone, M., Chatelain, L., Scopelliti, R., Zivkovic, I. & Mazzanti, M. Nitrogen reduction and functionalization by a multimetallic uranium nitride complex. Nature 547, 332–335 (2017).
Anderson, J. S., Rittle, J. & Peters, J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–87 (2013).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
MacLeod, K. C. & Holland, P. L. Recent developments in the homogeneous reduction of dinitrogen by molybdenum and iron. Nat. Chem. 5, 559–565 (2013).
Haber, F. Verfahren zur Herstellung von Ammoniak durch katalytische Vereinigung von Stickstoff und Wasserstoff, zweckmäßig unter hohem Druch. German patent DE 229126 (1909).
Cloke, G. F. N. & Hitchcock, P. B. Reversible binding and reduction of dinitrogen by a uranium (iii) pentalene complex. J. Am. Chem. Soc. 124, 9352–9353 (2002).
Odom, A. L., Arnold, P. L. & Cummins, C. C. Heterodinuclear uranium/molybdenum dinitrogen complexes. J. Am. Chem. Soc. 120, 5836–5837 (1998).
Mansell, S. M., Kaltsoyannis, N. & Arnold, P. L. Small molecule activation by uranium tris(aryloxides): experimental and computational studies of binding of N2, coupling of CO, and deoxygenation insertion of CO2 under ambient conditions. J. Am. Chem. Soc. 133, 9036–9051 (2011).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. A monometallic f element complex of dinitrogen: (C5Me5)3U(η1-N2). J. Am. Chem. Soc. 125, 14264–14265 (2003).
Roussel, P. & Scott, P. Complex of dinitrogen with trivalent uranium. J. Am. Chem. Soc. 120, 1070–1071 (1998).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. Bis(pentamethylcyclopentadienyl) U(iii) oxide and U(iv) oxide carbene complexes. Polyhedron 23, 2689–2694 (2004).
Larch, C. P., Cloke, F. G. N. & Hitchcock, P. B. Activation and reduction of diethyl ether by low valent uranium: formation of the trimetallic, mixed valence uranium oxo species [U(CpRR’)(µ-I)2]3(µ3-O) (CpRR’ = C5Me5, C5Me4H, C5H4SiMe3). Chem. Commun. 82–84 (2008).
Castro-Rodriguez, I., Olsen, K., Gantzel, P. & Meyer, K. Uranium complexes supported by an aryloxide functionalised triazacyclononane macrocycle: synthesis and characterisation of a six-coordinate U(iii) species and insights into its reactivity. Chem. Commun. 2764–2765 (2002).
Wang, J. X., Gurevich, Y., Botoshansky, M. & Eisen, M. S. Unique σ-bond metathesis of silylalkynes promoted by an ansa-dimethylsilyl and oxo-bridged uranium metallocene. J. Am. Chem. Soc. 128, 9350–9351 (2006).
Lam, O. P., Bart, S. C., Kameo, H., Heinemann, F. W. & Meyer, K. Insights into the mechanism of carbonate formation through reductive cleavage of carbon dioxide with low-valent uranium centers. Chem. Commun. 46, 3137–3139 (2010).
Castro, L., Lam, O. P., Bart, S. C., Meyer, K. & Maron, L. Carbonate formation from CO2 via oxo versus oxalate pathway: theoretical investigations into the mechanism of uranium-mediated carbonate formation. Organometallics 29, 5504–5510 (2010).
Fortier, S., Brown, J. L., Kaltsoyannis, N., Wu, G. & Hayton, T. W. Synthesis, molecular and electronic structure of UV(O)[N(SiMe3)2]3. Inorg. Chem. 51, 1625–1633 (2012).
Berthet, J. C. et al. Synthesis and crystal structure of the oxo-bridged bimetallic organouranium complex [{(Me3SiC5H4)3U2}µ-O]. J. Organomet. Chem. 408, 335–341 (1991).
Schmidt, A. C., Nizovtsev, A. V., Scheurer, A., Heinemann, F. W. & Meyer, K. Uranium-mediated reductive conversion of CO2 to CO and carbonate in a single-vessel, closed synthetic cycle. Chem. Commun. 48, 8634–8636 (2012).
Frey, A. S. P., Cloke, F. G. N., Coles, M. P. & Hitchcock, P. B. UIII-induced reductive co-coupling of NO and CO to form U(iv) cyanate and oxo derivates. Chem. Eur. J. 16, 9446–9448 (2010).
Avens, L. R., Barnhart, D. M., Burns, C. J., McKee, S. D. & Smith, W. H. Oxidation chemistry of a U(iii) aryloxide. Inorg. Chem. 33, 4245–4254 (1994).
Falcone, M., Kefalidis, C. E., Scopelliti, R., Maron, L. & Mazzanti, M. Facile CO cleavage by a multimetallic CsU2 nitride complex. Angew. Chem. Int. Ed. 55, 12290–12294 (2016).
Falcone, M., Chatelain, L. & Mazzanti, M. Nucleophilic reactivity of a nitride-bridged diuranium(iv) complex: CO2 and CS2 functionalization. Angew. Chem. Int. Ed. 55, 4074–4078 (2016).
Mougel, V. et al. Siloxides as supporting ligands in uranium(iii)-mediated small-molecule activation. Angew. Chem. Int. Ed. 51, 12280–12284 (2012).
Tskhovrebov, A. G., Vuichoud, B., Solari, E., Scopelliti, R. & Severin, K. Adducts of nitrous oxide and N-heterocyclic carbenes: syntheses, structures, and reactivity. J. Am. Chem. Soc. 135, 9486–9492 (2013).
Tskhovrebov, A. G., Solari, E., Wodrich, M. D., Scopelliti, R. & Severin, K. Covalent capture of nitrous oxide by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 51, 232–234 (2012).
Palluccio, T. D. et al. Thermodynamic and kinetic study of cleavage of the N–O bond of N-oxides by a vanadium(iii) complex: enhanced oxygen atom transfer reaction rates for adducts of nitrous oxide and mesityl nitrile oxide. J. Am. Chem. Soc. 135, 11357–11372 (2013).
Huynh, M. H. V., White, P. S., Carter, C. A. & Meyer, T. J. Formation and reactivity of the osmium(iv)–cyanoimido complex OsIV(bpy)(Cl)3(NCN)−. Angew. Chem. Int. Ed. 40, 3037–3039 (2001).
Kajitani, H., Tanabe, Y., Kuwata, S., Iwasaki, M. & Ishii, Y. A cyanamido-bridged diiridium complex: a reactive building block for polynuclear cyanamido complexes. Organometallics 24, 2251–2254 (2005).
Mindiola, D. J. et al. Bimetallic µ-cyanoimide complexes prepared by NCN group transfer. Chem. Commun. 125–126 (2001).
Kindra, D. R. & Evans, W. J. Magnetic susceptibility of uranium complexes. Chem. Rev. 114, 8865–8882 (2014).
Pool, J. A., Bernskoetter, W. H. & Chirik, P. J. On the origin of dinitrogen hydrogenation promoted by [(η5-C5Me4H)2Zr]2(µ2,η2,η2-N2). J. Am. Chem. Soc. 126, 14326–14327 (2004).
La Pierre, H. S. & Meyer, K. in Progress in Inorganic Chemistry Vol. 58 (ed. Karlin, K. D.) 303–415 (Wiley, Hoboken, 2014).
Fryzuk, M. D., Haddad, T. S. & Rettig, S. J. Reduction of dinitrogen by a zirconium phosphine complex to form a side-on-bridging N2 ligand. Crystal structure of {[(Pri 2PCH2SiMe2)2N]ZrCl}2(µ-η2:η2-N2). J. Am. Chem. Soc. 112, 8185–8186 (1990).
Ohki, Y. & Fryzuk, M. D. Dinitrogen activation by group 4 metal complexes. Angew. Chem. Int. Ed. 46, 3180–3183 (2007).
Tanabe, Y., Kuwata, S. & Ishii, Y. Syntheses and skeletal transformations of NCNH- and NCN-bridged tetrairidium(iii) cages. J. Am. Chem. Soc. 124, 6528–6529 (2002).
Schmidt, A.-C., Heinemann, F. W., Lukens, W. W. Jr & Meyer, K. Molecular and electronic structure of dinuclear uranium bis-μ-oxo complexes with diamond core structural motifs. J. Am. Chem. Soc. 136, 11980–11993 (2014).
Reynolds, J. G., Zalkin, A., Templeton, D. H. & Edelstein, N. M. Syntheses and crystal structures of tetrakis(diphenylamido)uranium(iv) and bis(µ-oxo-tris(diphenylamido)uranium(iv)lithium diethyl etherate. Inorg. Chem. 16, 1090–1096 (1977).
Acknowledgements
The authors acknowledge support from the Swiss National Science Foundation (grant nos. 200021_162430 and 200021_178793) and from the Ecole Polytechnique Fédérale de Lausanne (EPFL). The authors thank E. Solari for carrying out the elemental analyses, L. Y. M. Eymann for synthesis of the IMesN2O adduct and R. Scopelliti for important contributions to the X-ray single-crystal structure analyses.
Author information
Authors and Affiliations
Contributions
M.F. and L.B. carried out the synthetic experiments and analysed the experimental data. J.A. performed preliminary experiments and characterized the U–O–U complexes. F.F.T. carried out the X-ray single-crystal structure analyses. I.Z. measured and analysed the magnetic data. A.F. and C.C. performed the theoretical computations. K.S. contributed to the identification of a new route to oxo complexes. M.M. originated the central idea, coordinated the work and analysed the experimental data. M.M., M.F. and L.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Characterization, Supplementary Computational analysis
Crystallographic data
CIF for compound 2; CCDC reference: 1821630.
Crystallographic data
CIF for compound 3; CCDC reference: 1821631.
Crystallographic data
CIF for compound 4; CCDC reference: 1821633.
Crystallographic data
CIF for compound 5; CCDC reference: 1821634.
Crystallographic data
CIF for compound 6; CCDC reference: 1821632.
Rights and permissions
About this article
Cite this article
Falcone, M., Barluzzi, L., Andrez, J. et al. The role of bridging ligands in dinitrogen reduction and functionalization by uranium multimetallic complexes. Nature Chem 11, 154–160 (2019). https://doi.org/10.1038/s41557-018-0167-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0167-8
This article is cited by
-
Photochemical Synthesis of Transition Metal-Stabilized Uranium(VI) Nitride Complexes
Nature Communications (2022)
-
Metallacyclic actinide catalysts for dinitrogen conversion to ammonia and secondary amines
Nature Chemistry (2020)
-
Alternative Strategies Toward Sustainable Ammonia Synthesis
Transactions of Tianjin University (2020)