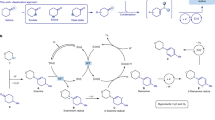
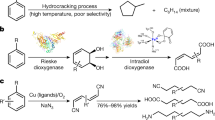
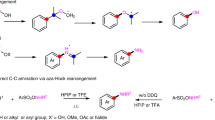
Abstract
Anilines are fundamental motifs in various chemical contexts, and are widely used in the industrial production of fine chemicals, polymers, agrochemicals and pharmaceuticals. A recent development for the synthesis of anilines uses the primary amination of C–H bonds in electron-rich arenes. However, there are limitations to this strategy: the amination of electron-deficient arenes remains a challenging task and the amination of electron-rich arenes has a limited control over regioselectivity—the formation of meta-aminated products is especially difficult. Here we report a site-directed C–C bond primary amination of simple and readily available alkylarenes or benzyl alcohols for the direct and efficient preparation of anilines. This chemistry involves a novel C–C bond transformation and offers a versatile protocol for the synthesis of substituted anilines. The use of O2 as an environmentally benign oxidant is demonstrated, and studies on model compounds suggest that this method may also be used for the depolymerization of lignin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Full experimental procedures and spectral data for all the new compounds as well as computational details are included in the Supplementary Information and are available from the corresponding authors on request.
Change history
08 November 2018
The version of this Article originally published online did not include a caution statement relating to safety concerns over some of the reagents used. All versions of the Article now have the following text included at the start of the Methods section “Caution: Sodium azide (NaN3) is highly toxic and also a potential explosion hazard; it can also react with organohalides to form explosive organic azides. Under acidic conditions, sodium azide can form hydrazoic acid (HN3) which is highly toxic. Considering these hazards, appropriate safety precautions should be taken when undertaking the C–C amination reactions reported in this Article.” Furthermore, in Fig. 2a, in the reaction conditions on the first equation it should have read DDQ not DDG; this has also been amended.
References
Lawrence, S. A. Amines: Synthesis, Properties and Applications (Cambridge Univ. Press, Cambridge, 2004).
Newhouse, T., Baran, P. S. & Hoffmann, R. W. The economies of synthesis. Chem. Soc. Rev. 38, 3010–3021 (2009).
Trost, B. M. Atom economy—a challenge for organic synthesis: homogeneous catalysis leads the way. Angew. Chem. Int. Ed. 34, 259–281 (1995).
Booth, G. Nitro Compounds, aromatic. In Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH, Weinheim, 2005).
Hartwig, J. F. Carbon–heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 31, 852–860 (1998).
Hartwig, J. F. Carbon–heteroatom bond formation catalyzed by organometallic complexes. Nature 455, 314–322 (2008).
Surry, D. S. & Buchwald, S. L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 47, 6338–6361 (2008).
Surry, D. S. & Buchwald, S. L. Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide. Chem. Sci. 2, 27–50 (2011).
Klinkenberg, J. L. & Hartwig, J. F. Catalytic organometallic reactions of ammonia. Angew. Chem. Int. Ed. 50, 86–95 (2011).
Gao, H. et al. Rapid heteroatom transfer to arylmetals utilizing multifunctional reagent scaffolds. Nat. Chem. 9, 681–688 (2017).
Tezuka, N. et al. Direct hydroxylation and amination of arenes via deprotonative cupration. J. Am. Chem. Soc. 138, 9166–9171 (2016).
Zhou, Z. et al. Non-deprotonative primary and secondary amination of (hetero)arylmetals. J. Am. Chem. Soc. 139, 115–118 (2017).
Morofuji, T., Shimizu, A. & Yoshida, J. Electrochemical C–H amination: synthesis of aromatic primary amines via N-arylpyridinium ions. J. Am. Chem. Soc. 135, 5000–5003 (2013).
Romero, N. A., Margrey., K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Zheng, Y.-W. et al. Photocatalytic hydrogen-evolution cross-couplings: benzene C–H amination and hydroxylation. J. Am. Chem. Soc. 138, 10080–10083 (2016).
Paudyal, M. P. et al. Dirhodium-catalyzed C–H arene aminaton using hydroxylamines. Science 353, 1144–1147 (2016).
Jones, W. D. The fall of the C–C bond. Nature 364, 676–677 (1993).
Gozin, M., Weisman, A., Ben-David, Y. & Milstein, D. Activation of a carbon–carbon bond in solution by transition-metal insertion. Nature 364, 699–701 (1993).
Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017).
Murakami, M., Amii, H. & Ito, Y. Selective activation of carbon–carbon bonds next to a carbonyl group. Nature 370, 540–541 (1994).
Masarwa, A. et al. Merging allylic carbon–hydrogen and selective carbon–carbon bond activation. Nature 505, 199–203 (2014).
Murphy, S. K., Park, J.-W., Cruz, F. A. & Dong., V. M. Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 347, 56–60 (2015).
Xia, Y., Lu, G., Liu, P. & Dong, G. Catalytic activation of carbon–carbon bonds in cyclopentanones. Nature 539, 546–550 (2016).
Bender, M., Turnbull, B. W. H., Ambler, B. R. & Krische, M. J. Ruthenium-catalyzed insertion of adjacent diol carbon atoms into C–C bonds: entry to type II polyketides. Science 357, 779–781 (2017).
Murakami, M. & Ishida, N. Potential of metal-catalyzed C–C single bond cleavage for organic synthesis. J. Am. Chem. Soc. 138, 13759–13769 (2016).
Hock, H. & Lang, S. Autoxydation von Kohlenwasserstoffen, IX. Mitteil.: Über Peroxyde von Benzol-Derivaten. Ber. Dtsch. Chem. Ges. B77, 257–264 (1944).
McMillen, D. F. & Golden, D. M. Hydrocarbon bond dissociation energies. Ann. Rev. Phys. Chem. 33, 493–532 (1982).
Brocks, J. J. et al. Estimation of bond dissociation energies and radical stabilization energies by ESR spectroscopy. J. Org. Chem. 63, 1935–1943 (1998).
Jun, C.-H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004).
Park, Y. J., Park, J.-W. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation and its concurrent recovery. Acc. Chem. Res. 41, 222–234 (2008).
Goossen, L. J., Rodríguez, N. & Goossen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 47, 3100–3120 (2008).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Dong, G. C–C Bond Activation (Topics in Current Chemistry 346, Springer, Berlin, 2014).
Tobisu, M. & Chatani, N. Catalytic reactions involving the cleavage of carbon–cyano and carbon–carbon triple bonds. Chem. Soc. Rev. 37, 300–307 (2008).
Morioka, T., Nishizawa, A., Furukawa, T., Tobisu, M. & Chatani, N. Nickel-mediated decarbonylation of simple unstrained ketones through the cleavage of carbon–carbon bonds. J. Am. Chem. Soc. 139, 1416–1419 (2017).
Ye, J. et al. Remote C–H alkylation and C–C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 9, 361–369 (2017).
Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014).
Rahimi, A., Azarpira, A., Kim, H., Ralph, J. & Stahl, S. S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 135, 6415–6418 (2013).
Qin, C., Shen, T., Tang., C. & Jiao, N. FeCl2-promoted cleavage of the unactivated C–C bond of alkylarenes and polystyrene: direct synthesis of arylamines. Angew. Chem. Int. Ed. 51, 6971–6975 (2012).
Wang, T. & Jiao, N. TEMPO-catalyzed aerobic oxygenation and nitrogenation of olefins via C=C double bond cleavage. J. Am. Chem. Soc. 135, 11692–11695 (2013).
Shen, T., Wang., T., Qin, C. & Jiao, N. Silver-catalyzed nitrogenation of alkynes: a direct approach to nitriles through C≡C bond cleavage. Angew. Chem. Int. Ed. 52, 6677–6680 (2013).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Bharadwaj, S. K., Boruah, P. K. & Gogoi, P. K. Phosphoric acid modified montmorillonite clay: a new heterogeneous catalyst for nitration of arenes. Catal. Commun. 57, 124–128 (2014).
Torker, S., Müller, A. & Chen, P. Building stereoselectivity into a chemoselective ring-opening metathesis polymerization catalyst for alternating copolymerization. Angew. Chem. Int. Ed. 49, 3762–3766 (2010).
Wang, P.-C., Yao, K. & Lu, M. Preparation of heteropoly acid based amphiphilic salts supported by nano oxides and their catalytic performance in the nitration of aromatics. RSC Adv. 3, 2197–2202 (2013).
Schweighauser, L., Strauss, M. A., Bellotto, S. & Wegner, H. A. Attraction or repulsion? London dispersion forces control azobenzene switches. Angew. Chem. Int. Ed. 54, 13436–13439 (2015).
Song, S. et al. Study on the design, synthesis and structure–activity relationships of new thiosemicarbazone compounds as tyrosinase inhibitors. Eur. J. Med. Chem. 139, 815–825 (2017).
West, J. D., Stafford, S. E. & Meter, M. P. A mechanistic probe for asymmetric reactions: deuterium isotope effects at enantiotopic groups. J. Am. Chem. Soc. 130, 7816–7817 (2008).
Mou, J., Park, A., Cai, Y., Yuan, J. & Yuan, C. Structure–activity relationship study of E6 as a novel necroptosis inducer. Bioorg. Med. Chem. Lett. 25, 3057–3061 (2015).
Acknowledgements
Financial support from the National Basic Research Program of China (973 Program) (no. 2015CB856600), the National Natural Science Foundation of China (nos 21632001 and 21772002) and Peking University Health Science Center (no. BMU20160541) are appreciated.
Author information
Authors and Affiliations
Contributions
J.L. and N.J. conceived and designed the experiments; J.L., X.Q. and C.Z. carried out most of experiments; J.L., X.Q., X.L., J.W., J.P. and N.J. analysed data; J.L., X.Q., X.H., J.W., J.P., Y.L., Y.Z., Q.Q., S.S. and N.J. participated in discussion and co-wrote the paper; N.J. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General information, optimization data, substrate synthesis, general procedures, synthetic application, mechanistic experiments, additional references and characterization data
Rights and permissions
About this article
Cite this article
Liu, J., Qiu, X., Huang, X. et al. From alkylarenes to anilines via site-directed carbon–carbon amination. Nature Chem 11, 71–77 (2019). https://doi.org/10.1038/s41557-018-0156-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0156-y
This article is cited by
-
Synthesis of meta-carbonyl phenols and anilines
Nature Communications (2024)
-
Metal-free visible light mediated direct C–H amination of benzoxazole with secondary amines
Molecular Diversity (2024)
-
Direct conversion of lignin to functionalized diaryl ethers via oxidative cross-coupling
Nature Communications (2023)
-
Mn(I)-catalyzed sigmatropic rearrangement of β, γ-unsaturated alcohols
Nature Communications (2023)
-
Towards bioresource-based aggregation-induced emission luminogens from lignin β-O-4 motifs as renewable resources
Nature Communications (2023)