Abstract
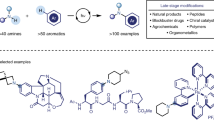
Synthetic chemistry is built around the formation of carbon–carbon bonds. However, the development of methods for selective carbon–carbon bond cleavage is a largely unmet challenge1,2,3,4,5,6. Such methods will have promising applications in synthesis, coal liquefaction, petroleum cracking, polymer degradation and biomass conversion. For example, aromatic rings are ubiquitous skeletal features in inert chemical feedstocks, but are inert to many reaction conditions owing to their aromaticity and low polarity. Over the past century, only a few methods under harsh conditions have achieved direct arene-ring modifications involving the cleavage of inert aromatic carbon–carbon bonds7,8, and arene-ring-cleavage reactions using stoichiometric transition-metal complexes or enzymes in bacteria are still limited9,10,11. Here we report a copper-catalysed selective arene-ring-opening reaction strategy. Our aerobic oxidative copper catalyst converts anilines, arylboronic acids, aryl azides, aryl halides, aryl triflates, aryl trimethylsiloxanes, aryl hydroxamic acids and aryl diazonium salts into alkenyl nitriles through selective carbon–carbon bond cleavage of arene rings. This chemistry was applied to the modification of polycyclic aromatics and the preparation of industrially important hexamethylenediamine and adipic acid derivatives. Several examples of the late-stage modification of complex molecules and fused ring compounds further support the potential broad utility of this methodology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
National Research Council (US) Health and Medicine: Challenges for the Chemical Sciences in the 21st Century (National Academies Press, 2004).
Jones, W. D. The fall of the C–C bond. Nature 364, 676–677 (1993).
Zhu, J., Wang, J. & Dong, G. Catalytic activation of unstrained C(aryl) –C(aryl) bonds in 2,2′-biphenols. Nat. Chem. 11, 45–51 (2019).
Guengerich, F. P. & Yoshimoto, F. K. Formation and cleavage of C–C Bonds by enzymatic oxidation–reduction reactions. Chem. Rev. 118, 6573–6655 (2018).
Jakoobi, M. & Sergeev, A. G. Transition-metal-mediated cleavage of C–C bonds in aromatic rings. Chem. Asian J. 14, 2181–2192 (2019).
Murakami, M. & Chatani, N. Cleavage of Carbon–Carbon Single Bonds by Transition Metals (Wiley, 2015).
Mortier, J. Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds (Wiley, 2015).
Benitez, V. M., Grau, J. M., Yori, J. C., Pieck, C. L. & Vera, C. R. Hydroisomerization of benzene-containing paraffinic feedstocks over Pt/WO3–ZrO2 catalysts. Energy Fuels 20, 1791–1798 (2006).
Sattler, A. & Parkin, G. Cleaving carbon–carbon bonds by inserting tungsten into unstrained aromatic rings. Nature 463, 523–526 (2010).
Hu, S., Shima, T. & Hou, Z. Carbon–carbon bond cleavage and rearrangement of benzene by a trinuclear titanium hydride. Nature 512, 413–415 (2014).
Bugg, T. D. H. & Winfield, C. J. Enzymatic cleavage of aromatic rings: mechanistic aspects of the catechol dioxygenases and later enzymes of bacterial oxidative cleavage pathways. Nat. Prod. Rep. 15, 513–530 (1998).
Wilson, J. Celebrating Michael Faraday’s discovery of benzene. Ambix 59, 241–265 (2013).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Wang, Y., Li, J. & Liu, A. Oxygen activation by mononuclear nonheme iron dioxygenases involved in the degradation of aromatics. J. Biol. Inorg. Chem. 22, 395–405 (2017).
Siddiqi, Z., Wertjes, W. C. & Sarlah, D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 142, 10125–10131 (2020).
Kong, R. Y. & Crimmin, M. R. Chemoselective C–C σ‐bond activation of the most stable ring in biphenylene. Angew. Chem. Int. Ed. 60, 2619–2623 (2021).
Nakagawa, K. & Onoue, H. Oxidation of o-phenylenediamines with lead tetra-acetate. Chem. Commun. (London) 396a (1965).
Nakagawa, K. & Onoue, H. Oxidation with nickel peroxide. V. The formation of cis,cis-1,4-dicyano-1,3-butadienes in the oxidation of o-phenylendiamines. Tetrahedr. Lett. 6, 1433–1436 (1965).
Kajimoto, T., Takahashi, H. & Tsuji, J. Copper-catalyzed oxidation of o-phenylenediamines to cis,cis-mucononitriles. J. Org. Chem. 41, 1389–1393 (1976).
Buchner, E. & Curtius, T. Synthese von Ketonsäureäthern aus Aldehyden und Diazoessigäther. Ber. Dtsch. Chem. Ges. 18, 2371–2377 (1885).
Chapman, O. L. & Leroux, J. P. 1-Aza-1,2,4,6-cycloheptatetraene. J. Am. Chem. Soc. 100, 282–285 (1978).
Satake, K., Mizushima, H., Kimura, M. & Morosawa, S. The reactions of nitrene for the conjugated π-systems. Heterocycles 23, 195 (1985).
Liu, L. L. et al. A transient vinylphosphinidene via a phosphirene-phosphinidene rearrangement. J. Am. Chem. Soc. 140, 147–150 (2018).
Hall, J. H. Dinitrenes from o-diazides. synthesis of 1,4-dicyano-1,3-butadienes. J. Am. Chem. Soc. 87, 1147–1148 (1965).
Campbell, C. D. & Rees, C. W. Oxidation of 1- and 2-aminobenzotriazole. Chem. Commun. (London) 192–193 (1965).
Nicolaides, A. et al. Of ortho-conjugatively linked reactive intermediates: the cases of ortho-phenylene-(bis)nitrene, -carbenonitrene, and -(bis)carbene. J. Am. Chem. Soc. 121, 10563–10572 (1999).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Chen, Y., Kamlet, A. S., Steinman, J. B. & Liu, D. R. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 3, 146–153 (2011).
Prescher, J. A., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Haag, S. M. et al. Targeting STING with covalent small-molecule inhibitors. Nature 559, 269–273 (2018).
Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Zhdankin, V. V. et al. Preparation, X-ray crystal structure, and chemistry of stable azidoiodinanes derivatives of benziodoxole. J. Am. Chem. Soc. 118, 5192–5197 (1996).
Huang, X., Bergsten, T. M. & Groves, J. T. Manganese-catalyzed late-stage aliphatic C–H azidation. J. Am. Chem. Soc. 137, 5300–5303 (2015).
Schmidt, K. F. Über die Einwirkung von NH auf organische Verbindungen. Angew. Chem. 36, 511 (1923).
Schmidt, K. F. Über den Imin-Rest. Ber. Dtsch. Chem. Ges. 57B, 704–706 (1924).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon–carbon amination. Nat. Chem. 11, 71–77 (2018).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Scriven, E. F. V. & Turnbull, K. Azides: their preparation and synthetic uses. Chem. Rev. 88, 297–368 (1988).
Lombardi, F. et al. Quantum units from the topological engineering of molecular graphenoids. Science 366, 1107–1110 (2019).
Kolmer, M. et al. Fluorine-programmed nanozipping to tailored nanographenes on rutile TiO2 surfaces. Science 363, 57–60 (2019).
Yano, Y. et al. Living annulative pi-extension polymerization for graphene nanoribbon synthesis. Nature 571, 387–392 (2019).
Hawker, C. J. & Wooley, K. L. The convergence of synthetic organic and polymer chemistries. Science 309, 1200–1205 (2005).
Oh, J. Y. et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016).
Bakhoda, A. G., Jiang, Q., Bertke, J. A., Cundari, T. R. & Warren, T. H. Elusive terminal copper arylnitrene intermediates. Angew. Chem. Int. Ed. 56, 6426–6430 (2017).
Carsch, K. M. et al. Synthesis of a copper-supported triplet nitrene complex pertinent to copper-catalyzed amination. Science 365, 1138–1143 (2019).
Allen, S. E., Walvoord, R. R., Padilla-Salinas, R. & Kozlowski, M. C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 113, 6234–6458 (2013).
McCann, S. D. & Stahl, S. S. Copper-catalyzed aerobic oxidations of organic molecules: pathways for two-electron oxidation with a four-electron oxidant and a one-electron redox-active catalyst. Acc. Chem. Res. 48, 1756–1766 (2015).
Acknowledgements
We acknowledge the NSFC (grant numbers 21632001, 21772002, 81821004, 21933004), the National Key Research and Development Project (grant number 2019YFC1708902), and the US National Science Foundation (CHE-1764328) for financial support of this research.
Author information
Authors and Affiliations
Contributions
N.J. conceived the project and directed the research. K.N.H. and X.-S.X. supervised the mechanistic study. X.Q., Y.S., X.-S.X., K.N.H. and N.J. wrote the paper. X.Q., H.W., Z.Y., Y.W., Z.C. and X.W. performed the experiments. Y.S. performed the DFT calculations. H.T, S.S., G.Z. and X.Z. discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Adrian Mulholland and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Downstream transformations and mechanism studies.
a, Downstream transformations of alkenyl nitriles. b, The excluded intermediates. c, HOMO(α) and HOMO-1(β) of the triplet copper bis-nitrene intermediate. *See Supplementary Information for experimental details.
Supplementary information
Supplementary Information
This file contains Supplementary Information (see Table of Contents in PDF for full description).
Supplementary Data
This file contains the crystal structure of 48 in Fig 3.
Rights and permissions
About this article
Cite this article
Qiu, X., Sang, Y., Wu, H. et al. Cleaving arene rings for acyclic alkenylnitrile synthesis. Nature 597, 64–69 (2021). https://doi.org/10.1038/s41586-021-03801-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03801-y
This article is cited by
-
Site-selective arene C–H amination with iron-aminyl radical
Nature Catalysis (2024)
-
Nanoconfinement-triggered oligomerization pathway for efficient removal of phenolic pollutants via a Fenton-like reaction
Nature Communications (2024)
-
C(alkyl)–C(vinyl) bond cleavage enabled by Retro-Pallada-Diels-Alder reaction
Nature Communications (2023)
-
Single-atom logic for heterocycle editing
Nature Synthesis (2022)
-
Benzene rings broken for chemical synthesis
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.