Abstract
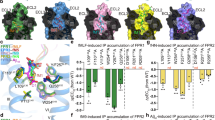
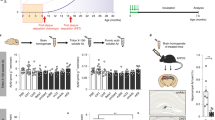
Inhibiting the interaction between amyloid-β (Aβ) and a neuronal cell surface receptor, LilrB2, has been suggested as a potential route for treating Alzheimer’s disease. Supporting this approach, Alzheimer’s-like symptoms are reduced in mouse models following genetic depletion of the LilrB2 homologue. In its pathogenic, oligomeric state, Aβ binds to LilrB2, triggering a pathway to synaptic loss. Here we identify the LilrB2 binding moieties of Aβ (16KLVFFA21) and identify its binding site on LilrB2 from a crystal structure of LilrB2 immunoglobulin domains D1D2 complexed to small molecules that mimic phenylalanine residues. In this structure, we observed two pockets that can accommodate the phenylalanine side chains of KLVFFA. These pockets were confirmed to be 16KLVFFA21 binding sites by mutagenesis. Rosetta docking revealed a plausible geometry for the Aβ–LilrB2 complex and assisted with the structure-guided selection of small molecule inhibitors. These molecules inhibit Aβ–LilrB2 interactions in vitro and on the cell surface and reduce Aβ cytotoxicity, which suggests these inhibitors are potential therapeutic leads against Alzheimer’s disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The crystal structure reported here, LilrB2 D1D2 complexed with benzamidine, and the corresponding diffraction data have been deposited to the Protein Data Bank (PDB) with the accession code 6BCS. All other data are available upon reasonable request to the authors.
Change history
12 November 2018
In the version of this Article originally published online, the upper right panel of Fig. 5a was mistakenly a repeat of the lower right panel. This has now been corrected in all versions of the Article.
References
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Sheng, M., Sabatini, B. L. & Sudhof, T. C. Synapses and Alzheimer’ disease. Cold Spring Harb. Perspect. Biol. 4, a005777 (2012).
Braak, H. & Braak, E. Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J. Neuropathol. Exp. Neurol. 49, 215–224 (1990).
Nagy, Z. et al. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience 69, 757–761 (1995).
Seidler, P. M. et al. Structure-based inhibitors of tau aggregation. Nat. Chem. 10, 170–176 (2018).
Tanzi, R. E. & Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120, 545–555 (2005).
Bettens, K., Sleegers, K. & Van Broeckhoven, C. Current status on Alzheimer disease molecular genetics: from past, to present, to future. Hum. Mol. Genet. 19, R4–R11 (2010).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Li, S. et al. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 31, 6627–6638 (2011).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Ashe, K. H. & Zahs, K. R. Probing the biology of Alzheimer’s disease in mice. Neuron 66, 631–645 (2010).
Sorrentino, V. et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552, 187–193 (2017).
Zhu, B. et al. ER-associated degradation regulates Alzheimer’s amyloid pathology and memory function by modulating ɣ-secretase activity. Nat. Commun. 8, 1472 (2017).
Cheng, P. N., Liu, C., Zhao, M., Eisenberg, D. & Nowick, J. S. Amyloid β-sheet mimics that antagonize protein aggregation and reduce amyloid toxicity. Nat. Chem. 4, 927–933 (2012).
Man, B. Y.-W. et al. Group 9 metal-based inhibitors of β-amyloid (1–40) fibrillation as potential therapeutic agents for Alzheimer’s disease. Chem. Sci. 2, 917 (2011).
Ehrnhoefer, D. E. et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 15, 558–566 (2008).
Dovey, H. F. et al. Functional ɣ-secretase inhibitors reduce β-amyloid peptide levels in brain. J. Neurochem. 76, 173–181 (2001).
Evin, G. Future therapeutics in Alzheimer’s disease: development status of BACE inhibitors. BioDrugs 30, 173–194 (2016).
Mitani, Y. et al. Differential effects between ɣ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J. Neurosci. 32, 2037–2050 (2012).
Castillo-Carranza, D. L., Guerrero-Munoz, M. J. & Kayed, R. Immunotherapy for the treatment of Alzheimer’s disease: amyloid-β or τ, which is the right target? Immunotargets Ther. 3, 19–28 (2014).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Lauren, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Cisse, M. et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469, 47–52 (2011).
Jarosz-Griffiths, H. H., Noble, E., Rushworth, J. V. & Hooper, N. M. Amyloid-β receptors: the good, the bad, and the prion protein. J. Biol. Chem. 291, 3174–3183 (2016).
Kim, T. et al. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science 341, 1399–1404 (2013).
Luhrs, T. et al. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl Acad. Sci. USA 102, 17342–17347 (2005).
Colletier, J. P. et al. Molecular basis for amyloid-β polymorphism. Proc. Natl Acad. Sci. USA 108, 16938–16943 (2011).
Willcox, B. E. et al. Crystal structure of LIR-2 (ILT4) at 1.8 Å: differences from LIR-1 (ILT2) in regions implicated in the binding of the human cytomegalovirus class I MHC homolog UL18. BMC Struct. Biol. 2, 6 (2002).
Raveh, B., London, N., Zimmerman, L. & Schueler-Furman, O. Rosetta FlexPepDock ab-initio: simultaneous folding, docking and refinement of peptides onto their receptors. PLoS ONE 6, e18934 (2011).
Stroud, J. C., Liu, C., Teng, P. K. & Eisenberg, D. Toxic fibrillar oligomers of amyloid-β have cross-β structure. Proc. Natl Acad. Sci. USA 109, 7717–7722 (2012).
Kuhlman, B. & Baker, D. Native protein sequences are close to optimal for their structures. Proc. Natl Acad. Sci. USA 97, 10383–10388 (2000).
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993).
Jiang, L. et al. Structure-based discovery of fiber-binding compounds that reduce the cytotoxicity of amyloid β. eLife 2, e00857 (2013).
Huang, H. C., Chang, P., Dai, X. L. & Jiang, Z. F. Protective effects of curcumin on amyloid-β-induced neuronal oxidative damage. Neurochem. Res. 37, 1584–1597 (2012).
He, Y. et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (−)-epigallocatechin-3-gallate. ASN Neuro. 3, e00050 (2011).
Syken, J., Grandpre, T., Kanold, P. O. & Shatz, C. J. PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800 (2006).
Gremer, L. et al. Fibril structure of amyloid-β(1–42) by cryo-electron microscopy. Science 358, 116–119 (2017).
Liu, C. et al. Out-of-register β-sheets suggest a pathway to toxic amyloid aggregates. Proc. Natl Acad. Sci. USA 109, 20913–20918 (2012).
Laganowsky, A. et al. Atomic view of a toxic amyloid small oligomer. Science 335, 1228–1231 (2012).
Nalivaeva, N. N., Belyaev, N. D., Kerridge, C. & Turner, A. J. Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer’s disease. Front. Aging Neurosci. 6, 235 (2014).
Crespi, G. A., Hermans, S. J., Parker, M. W. & Miles, L. A. Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci. Rep. 5, 9649 (2015).
Abbott, A. & Dolgin, E. Failed Alzheimer’s trial does not kill leading theory of disease. Nature 540, 15–16 (2016).
Wang, S. et al. D4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science 358, 381–386 (2017).
Acknowledgements
The authors thank C. Shatz for providing the LilrB2 plasmid. This work is based on research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P41 GM103403). The Pilatus 6M detector on the 24-ID-C beamline is funded by a NIH-ORIP HEI grant (S10 RR029205). This research used resources from the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. L.J. is supported by UCLA departmental recruitment funds. The authors also acknowledge NIH AG 054022 and DOE DE-FC02-02ER63421 for support.
Author information
Authors and Affiliations
Contributions
Q.C., D.S.E. and L.J. conceived and designed the experiments. Q.C., W.S.S., H.C., C.K.V., B.D. and B.L. performed the experiments. W.S.S., B.D., K.A.M. and L.J. performed computational docking and structure-guided selection of small molecules. H.C. and J.F. performed and analysed NMR experiments. B.L. and L.J. performed and analysed circular dichroism experiments. C.K.V. and D.L.B. cultured primary neurons. Q.C. and M.R.S. solved the structure of the LilrB2 and benzamidine complex. All authors discussed the results and commented on the manuscript. Q.C., D.S.E. and L.J. analysed the data and co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
D.S.E. is an advisor and equity shareholder in ADDRx.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods and materials, Supplementary Figures 1–7, and Supplementary Table 1–4
Rights and permissions
About this article
Cite this article
Cao, Q., Shin, W.S., Chan, H. et al. Inhibiting amyloid-β cytotoxicity through its interaction with the cell surface receptor LilrB2 by structure-based design. Nature Chem 10, 1213–1221 (2018). https://doi.org/10.1038/s41557-018-0147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0147-z
This article is cited by
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases
Molecular Neurodegeneration (2024)
-
Simulations of Amyloid-Forming Peptides in the Crystal State
The Protein Journal (2023)
-
LILRB2-mediated TREM2 signaling inhibition suppresses microglia functions
Molecular Neurodegeneration (2022)
-
LOTUS suppresses amyloid β-induced dendritic spine elimination through the blockade of amyloid β binding to PirB
Molecular Medicine (2022)
-
Recognition of Aβ oligomer by LilrB2 acceptor: a tetracoordinated zipper mechanism
Journal of Molecular Modeling (2022)