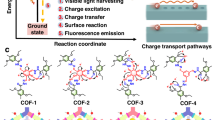
Abstract
Nature uses organic molecules for light harvesting and photosynthesis, but most man-made water splitting catalysts are inorganic semiconductors. Organic photocatalysts, while attractive because of their synthetic tunability, tend to have low quantum efficiencies for water splitting. Here we present a crystalline covalent organic framework (COF) based on a benzo-bis(benzothiophene sulfone) moiety that shows a much higher activity for photochemical hydrogen evolution than its amorphous or semicrystalline counterparts. The COF is stable under long-term visible irradiation and shows steady photochemical hydrogen evolution with a sacrificial electron donor for at least 50 hours. We attribute the high quantum efficiency of fused-sulfone-COF to its crystallinity, its strong visible light absorption, and its wettable, hydrophilic 3.2 nm mesopores. These pores allow the framework to be dye-sensitized, leading to a further 61% enhancement in the hydrogen evolution rate up to 16.3 mmol g−1 h−1. The COF also retained its photocatalytic activity when cast as a thin film onto a support.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 1818058 (fused sulfone diamine, FSA) and 1818059 (sulfone diamine, SA). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available within the Article and its Supplementary Information and/or from the corresponding authors upon reasonable request.
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Sivula, K. & van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 1, 15010 (2016).
Chen, S., Takata, T. & Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2, 17050 (2017).
Wang, X. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009).
Sprick, R. S. et al. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 137, 3265–3270 (2015).
Zhang, G., Lan, Z.-A. & Wang, X. Conjugated polymers: catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 55, 2–18 (2016).
Yanagida, S., Kabumoto, A., Mizumoto, K., Pac, C. & Yoshino, K. Poly(para)phenylene-catalyzed photoreduction of water to hydrogen. Chem. Commun. 8, 474–475 (1985).
Shibata, T. et al. Novel visible-light-driven photocatalyst. Poly(p-phenylene)-catalyzed photoreductions of water, carbonyl compounds, and olefins. J. Phys. Chem. 94, 2068–2076 (1990).
Schwinghammer, K. et al. Crystalline carbon nitride nanosheets for improved visible-light hydrogen evolution. J. Am. Chem. Soc. 136, 1730–1733 (2014).
Schwab, M. G. et al. Photocatalytic hydrogen evolution through fully conjugated poly(azomethine) networks. Chem. Commun. 46, 8932 (2010).
Yang, C. et al. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 55, 9202–9206 (2016).
Li, L. et al. Rational design of porous conjugated polymers and roles of residual palladium for photocatalytic hydrogen production. J. Am. Chem. Soc. 138, 7681–7686 (2016).
Woods, D. J., Sprick, R. S., Smith, C. L., Cowan, A. J. & Cooper, A. I. A solution-processable polymer photocatalyst for hydrogen evolution from water. Adv. Energy Mater. 7, 1700479 (2017).
Sprick, R. S. et al. Extended conjugated microporous polymers for photocatalytic hydrogen evolution from water. Chem. Commun. 52, 10008–10011 (2016).
Sprick, R. S. et al. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem. Int. Ed. 55, 1792–1796 (2016).
Wang, k et al. Covalent triazine frameworks via a low temperature polycondensation approach. Angew. Chem. Int. Ed. 56, 14337–14341 (2017).
Meier, C. B. et al. Structure–property relationships for covalent triazine-based frameworks: the effect of spacer length on photocatalytic hydrogen evolution from water. Polymer 126, 283–290 (2017).
Bi, J. et al. Covalent triazine-based frameworks as visible light photocatalysts for the splitting of water. Macromol. Rapid Commun. 36, 1799–1805 (2015).
Zhang, G., Lan, Z.-A., Lin, L., Lin, S. & Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 7, 3062–3066 (2016).
Wang, L. et al. Conjugated microporous polymer nanosheets for overall water splitting using visible light. Adv. Mater. 29, 1702428 (2017).
Zhang, G. et al. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem. Int. Ed. 56, 13445–13449 (2017).
Coropceanu, V. et al. Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
Ockwig, N. W., Cote, A. P., Keeffe, M. O., Matzger, A. J. & Yaghi, O. M. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1171 (2005).
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Spitler, E. L. et al. Lattice expansion of highly oriented 2D phthalocyanine covalent organic framework films. Angew. Chem. Int. Ed. 51, 2623–2627 (2012).
Spitler, E. L. & Dichtel, W. R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2, 672–677 (2010).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012).
Huang, N., Wang, P. & Jiang, D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Wan, S. et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23, 4094–4097 (2011).
Thote, J. et al. A covalent organic framework–cadmium sulfide hybrid as a prototype photocatalyst for visible-light-driven hydrogen production. Chem. Eur. J. 20, 15961–15965 (2014).
Zhou, J. et al. A (001) dominated conjugated polymer with high-performance of hydrogen evolution under solar light irradiation. Chem. Commun. 53, 10536–10539 (2017).
Stegbauer, L., Schwinghammer, K. & Lotsch, B. V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 5, 2789–2793 (2014).
Vyas, V. S. et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 6, 8508 (2015).
Haase, F. et al. Structure–property–activity relationships in a pyridine containing azine-linked covalent organic framework for photocatalytic hydrogen evolution. Faraday Discuss. 162, 165–169 (2017).
Pachfule, P. et al. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc. 140, 1423–1427 (2018).
Banerjee, T. et al. Single site photocatalytic H2 evolution from covalent organic frameworks with molecular cobaloxime co-catalysts. J. Am. Chem. Soc. 139, 16228–16234 (2017).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Sick, T. et al. Oriented films of conjugated 2D covalent organic frameworks as photocathodes for water splitting. J. Am. Chem. Soc. 140, 2085–2092 (2018).
Zhu, Y. & Zhang, W. Reversible tuning of pore size and CO2 adsorption in azobenzene functionalized porous organic polymers. Chem. Sci. 5, 4957–4961 (2014).
Kruczynski, L. et al. Porous titania glass as a photocatalyst for hydrogen production from water. Nature 291, 399–401 (1981).
Wagner, F. T. & Somorjai, G. A. Photocatalytic hydrogen production from water on Pt-free SrTiO3 in alkali hydroxide solutions. Nature 285, 559–560 (1980).
Corp, K. L., Schlenker, C. W., Corp, K. L. & Schlenker, C. W. Ultrafast spectroscopy reveals electron transfer cascade that improves hydrogen evolution with carbon nitride photocatalysts. J. Am. Chem. Soc. 139, 7904–7912 (2017).
Kroeze, J. E., Savenije, T. J., Vermeulen, M. J. W. & Warman, J. M. Contactless determination of the photoconductivity action spectrum, exciton diffusion length, and charge separation efficiency in polythiophene-sensitized TiO2 bilayers. J. Phys. Chem. B 107, 7696–7705 (2003).
Bruno, A., Reynolds, L. X., Dyer-Smith, C., Nelson, J. & Haque, S. A. Determining the exciton diffusion length in a polyfluorene from ultrafast fluorescence measurements of polymer/fullerene blend films. J. Phys. Chem. C 117, 19832–19838 (2013).
Shaw, P. E., Ruseckas, A. & Samuel, I. D. W. Exciton diffusion measurements in poly(3-hexylthiophene). Adv. Mater. 20, 3516–3520 (2008).
Tezuka, Y., Fukushima, A., Matsui, S. & Imai, K. Surface studies on poly(vinyl alcohol)-poly(dimethylsiloxane) graft copolymers. J. Colloid Interface Sci. 114, 16–25 (1986).
Biswal, B. P. et al. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption. J. Mater. Chem. A 3, 23664–23669 (2015).
Guiglion, P., Butchosa, C. & Zwijnenburg, M. A. Polymer photocatalysts for water splitting: insights from computational modeling. Macromol. Chem. Phys. 217, 344–353 (2016).
Guiglion, P., Monti, A. & Zwijnenburg, M. A. Validating a density functional theory approach for predicting the redox potentials associated with charge carriers and excitons in polymeric photocatalysts. J. Phys. Chem. C 121, 1498–1506 (2017).
Bach, U. et al. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 395, 583–585 (1998).
Willkomm, J. et al. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 45, 9–23 (2016).
Abe, R., Sayama, K., Domen, K. & Arakawa, H. A new type of water splitting system composed of two different TiO2 photocatalysts (anatase, rutile) and a IO3 −/I− shuttle redox mediator. Chem. Phys. Lett. 344, 339–344 (2001).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Tada, H., Mitsui, T., Kiyonaga, T., Akita, T. & Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 5, 782–786 (2006).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018).
Schröder, M. et al. Hydrogen evolution reaction in a large-scale reactor using a carbon nitride photocatalyst under natural sunlight irradiation. Energy Technol. 3, 1014–1017 (2015).
Slater, A. G. & Cooper, A. I. Function-led design of new porous materials. Science 348, aaa8075 (2015).
Evans, A. M. et al. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 361, 52–57 (2018).
Teets, T. S. & Nocera, D. G. Photocatalytic hydrogen production. Chem. Commun. 47, 9268–9274 (2011).
Hisatomi, T., Kubota, J. & Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535 (2014).
Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Stephens, P., Devlin, F., Chabalowski, C. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Frisch, M. J. et al. Gaussian 16 revision A.03 (Gaussian, 2016).
Hirata, S. & Head-Gordon, M. Time-dependent density functional theory within the Tamm–Dancoff approximation. Chem. Phys. Lett. 314, 291–299 (1999).
Scalmani, G. & Frisch, M. J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 132, 114110 (2010).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 32, 1456 (2011).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Heyd, J. & Scuseria, G. E. Efficient hybrid density functional calculations in solids: assessment of the Heyd-Scuseria-Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 121, 1187–1192 (2004).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Erratum: ‘Hybrid functionals based on a screened Coulomb potential’. J. Chem. Phys. 124, 219906 (2006).
Butler, K. T., Hendon, C. H. & Walsh, A. Electronic chemical potentials of porous metal–organic frameworks. J. Am. Chem. Soc. 136, 2703–2706 (2014).
Acknowledgements
The authors acknowledge funding from the Engineering and Physical Sciences Research Council (EPSRC) (EP/N004884/1), the European Union’s Seventh Framework Programme through grant agreement nos. 321156 (ERC-AG-PE5-ROBOT) and 692685, and the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design. X.W. thanks the China Scholarship Council for a PhD studentship. Y.W. and W.-H.Z. acknowledge financial support from the NSFC for Creative Research Groups (21421004) and Key Project (21636002), NSFC/China and a Shanghai Oriental Scholarship. The authors thank M. Bilton for help with HR-TEM, F. McBride for help with AFM, and G.-H. Ning and H. Niu for useful discussions. The authors acknowledge the Diamond Light Source for access to beamlines I19 (MT15777) and I11 (EE12336), the ARCHER UK National Supercomputing Service, access provided via a Programme Grant (EP/N004884/1) and the EPSRC funded UK Materials Chemistry Consortium (EP/L000202/1), and the use of the facilities of the N8 HPC Centre of Excellence, provided and funded by the N8 Research Partnership and EPSRC (EP/K000225/1).
Author information
Authors and Affiliations
Contributions
A.I.C. and X.W. conceived the project. X.W. synthesized the COFs and performed the characterization and photocatalysis experiments. L.C. and M.A.Z. conceived the modelling strategy and performed the calculations. S.Y.C. carried out PXRD analyses. M.A.L. carried out single-crystal X-ray structure analysis. R.S.S. performed the TCSPC experiments and co-supervised, with A.I.C., the work on COF synthesis, characterization and photocatalysis. Y.Y. collected the water sorption isotherms. R.C., X.W. and L.C. interpreted the gas sorption isotherms. Y.W. and W.-H.Z. synthesized and characterized the WS5F dye. All authors interpreted the data and contributed to preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, Supplementary Characterization, Supplementary Figures 1–95; Supplementary References 1–15
Crystallographic data
CIF for SA compound; CCDC reference: 1818059
Crystallographic data
CIF for FSA compound; CCDC reference: 1818058
Computational data
Data from computational analysis of COF models and cluster models
Video
Video showing hydrogen evolution from a FS-COF thin film
Rights and permissions
About this article
Cite this article
Wang, X., Chen, L., Chong, S.Y. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nature Chem 10, 1180–1189 (2018). https://doi.org/10.1038/s41557-018-0141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0141-5
This article is cited by
-
Dynamic two-dimensional covalent organic frameworks
Nature Chemistry (2024)
-
Linkage conversions in single-crystalline covalent organic frameworks
Nature Chemistry (2024)
-
Multiscale structural engineering of cobalt single-atom catalyst for highly efficient photocatalytic CO2 reduction
Science China Materials (2024)
-
Metal-free 2-isocyanobiaryl-based cyclization reactions: phenanthridine framework synthesis
Molecular Diversity (2024)
-
Overcoming small-bandgap charge recombination in visible and NIR-light-driven hydrogen evolution by engineering the polymer photocatalyst structure
Nature Communications (2024)