Abstract
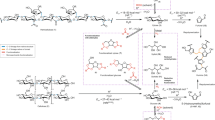
Polysaccharide depolymerization is an essential step for valorizing lignocellulosic biomass. In inexpensive systems such as pure water or dilute acid mixtures, carbohydrate monomer degradation rates exceed hemicellulose—and especially cellulose—depolymerization rates at most easily accessible temperatures, limiting sugar yields. Here, we use a reversible stabilization of xylose and glucose by acetal formation with formaldehyde to alter this kinetic paradigm, preventing sugar dehydration to furans and their subsequent degradation. During a harsh organosolv pretreatment in the presence of formaldehyde, over 90% of xylan in beech wood was recovered as diformylxylose (compared to 16% xylose recovery without formaldehyde). The subsequent depolymerization of cellulose led to carbohydrate yields over 70% and a final concentration of ~5 wt%, whereas the same conditions without formaldehyde gave a yield of 28%. This stabilization strategy pushes back the longstanding kinetic limits of polysaccharide depolymerization and enables the recovery of biomass-derived carbohydrates in high yields and concentrations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Luterbacher, J. S. et al. Solvent-enabled nonenyzmatic sugar production from biomass for chemical and biological upgrading. ChemSusChem 8, 1317–1322 (2015).
Luterbacher, J. S., Alonso, D. M. & Dumesic, J. A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green. Chem. 16, 4816–4838 (2014).
Questell-Santiago, Y. M. & Luterbacher, J. S. in High Pressure Technologies in Biomass Conversion (ed. Lukasik, R. M.) 9–36 (Royal Society of Chemistry, Croydon, 2017).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Wyman, C. E., Cai, C. M. & Kumar, R. in Encyclopedia of Sustainability Science and Technology (ed. Meyers, R. A.) 1–27 (Springer, New York, 2017).
Kumar, A. K. & Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4, 7 (2017).
Alonso, D. M. et al. Increasing the revenue from lignocellulosic biomass: maximizing feedstock utilization. Sci. Adv. 3, e1603301 (2017).
Lan, W., Amiri, M. T., Hunston, C. M. & Luterbacher, J. S. Protection group effects during α,γ-diol lignin stabilization promote high-selectivity monomer production. Angew. Chem. Int. Ed. 57, 1356–1360 (2018).
Nyoo Putro, J., Edi Soetaredjo, F., Lin, S.-Y., Ju, Y.-H. & Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 6, 46834–46852 (2016).
Luterbacher, J. S. et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014).
Haghighi Mood, S. et al. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 27, 77–93 (2013).
Neuman, R. P. & Walker, L. P. Solute exclusion from cellulose in packed columns: experimental investigation and pore volume measurements. Biotechnol. Bioeng. 40, 218–225 (1992).
Peterson, A. A. et al. Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ. Sci. 1, 32–65 (2008).
Bergius, F. Conversion of wood to carbohydrates. Ind. Eng. Chem. 29, 247–253 (1937).
Binder, J. B. & Raines, R. T. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl Acad. Sci. USA 107, 4516–4521 (2010).
Tao, L. et al. NREL 2012 Achievement of Ethanol Cost Targets: Biochemical Ethanol Fermentation via Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover (NREL, 2014); https://doi.org/10.2172/1129271
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A. & Blanch, H. W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 109, 1083–1087 (2012).
Gschwend, F. J. V., Brandt-Talbot, A., Chambon, C. L. & Hallett, J. P. in Ionic Liquids: Current State and Future Directions Vol. 1250 (eds Shiflett, M. B. & Scurto, A. M.) 209–223 (American Chemical Society, Washington DC, 2017).
Moriarty, K. L., Milbrandt, A. R., Warner, E., Lewis, J. E. & Schwab, A. A. 2016 Bioenergy Industry Status Report (NREL, Golden, 2018).
Liu, G., Zhang, J. & Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess. Biosyst. Eng. 39, 133–140 (2016).
Mellmer, M. A. et al. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. 53, 11872–11875 (2014).
Mellmer, M. A., Alonso, D. M., Luterbacher, J. S., Gallo, J. M. R. & Dumesic, J. A. Effects of γ-valerolactone in hydrolysis of lignocellulosic biomass to monosaccharides. Green Chem. 16, 4659–4662 (2014).
Ghosh, A., Bai, X. & Brown, R. C. Solubilized carbohydrate production by acid-catalyzed depolymerization of cellulose in polar aprotic solvents. Chem. Select 3, 4777–4785 (2018).
Lee, Y. Y., Iyer, P. & Torget, R. W. in Recent Progress in Bioconversion of Lignocellulosics (ed. Tsao, G. T.) 93–115 (Springer, Berlin, 1999).
Yang, B., Tao, L. & Wyman, C. E. Strengths, challenges, and opportunities for hydrothermal pretreatment in lignocellulosic biorefineries. Biofuels Bioprod. Bioref. 12, 125–138 (2018).
Han, J., Luterbacher, J. S., Alonso, D. M., Dumesic, J. A. & Maravelias, C. T. A lignocellulosic ethanol strategy via nonenzymatic sugar production: process synthesis and analysis. Bioresour. Technol. 182, 258–266 (2015).
Eerhart, A. J. J. E. et al. Fuels and plastics from lignocellulosic biomass via the furan pathway; a technical analysis. RSC Adv. 4, 3536–3549 (2013).
Eerhart, A. J. J. E., Patel, M. K. & Faaij, A. P. C. Fuels and plastics from lignocellulosic biomass via the furan pathway: an economic analysis. Biofuels Bioprod. Bioref. 9, 307–325 (2015).
Qian, M., Liauw, M. A. & Emig, G. Formaldehyde synthesis from methanol over silver catalysts. Appl. Catal. Gen. 238, 211–222 (2003).
Bahmanpour, A. M., Hoadley, A. & Tanksale, A. Formaldehyde production via hydrogenation of carbon monoxide in the aqueous phase. Green Chem. 17, 3500–3507 (2015).
Shuai, L. & Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 9, 133–155 (2016).
Mössinger, D., Scheytt, H., Uihlein, K., Wunderlich, D. & Zimmerer, B. High-tenacity viscose multifilament yarn with low yarn linear density. US Patent US20150322595A1 (2015).
Ronald, R., Martin, R. T. & Richardson, W. C. Filaments of regenerated cellulose. US Patent US3388117A (1968).
Pagán-Torres, Y. J., Wang, T., Gallo, J. M. R., Shanks, B. H. & Dumesic, J. A. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal. 2, 930–934 (2012).
Choudhary, V., Sandler, S. I. & Vlachos, D. G. Conversion of xylose to furfural using lewis and brønsted acid catalysts in aqueous media. ACS Catal. 2, 2022–2028 (2012).
Román-Leshkov, Y., Moliner, M., Labinger, J. A. & Davis, M. E. Mechanism of glucose isomerization using a solid Lewis acid catalyst in water. Angew. Chem. Int. Ed. 49, 8954–8957 (2010).
Choudhary, V., Pinar, A. B., Sandler, S. I., Vlachos, D. G. & Lobo, R. F. Xylose isomerization to xylulose and its dehydration to furfural in aqueous media. ACS Catal. 1, 1724–1728 (2011).
Robyt, J. F. Essentials of Carbohydrate Chemistry (Springer, New York, 1998).
Nimlos, M. R., Qian, X., Davis, M., Himmel, M. E. & Johnson, D. K. Energetics of xylose decomposition as determined using quantum mechanics modeling. J. Phys. Chem. A 110, 11824–11838 (2006).
Danon, B., Marcotullio, G. & Jong, Wde Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem. 16, 39–54 (2013).
Acknowledgements
This work was supported by the Swiss National Science Foundation through grant PYAPP2_154281, by the Swiss Competence Center for Energy Research: Biomass for a Swiss Energy Future through the Swiss Commission for Technology and Innovation grant KTI.2014.0116, and by EPFL. The authors thank M. Studer from Bern University of Applied Sciences (Switzerland) for providing beech wood.
Author information
Authors and Affiliations
Contributions
Y.M.Q.S. and J.S.L. conceived of the idea, designed the study and wrote the manuscript. Y.M.Q.S performed all experiments unless noted otherwise, and analysed the data. R.Z.V. performed xylose and diformylxylose dehydration reactions, and assisted in purifying diformylxylose and diformylglucose. M.T.A produced some of the pretreated solids and performed the preliminary techno-economic calculations. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary materials and methods, supplementary text, supplementary figures 1–22 and tables 1–10
Rights and permissions
About this article
Cite this article
Questell-Santiago, Y.M., Zambrano-Varela, R., Talebi Amiri, M. et al. Carbohydrate stabilization extends the kinetic limits of chemical polysaccharide depolymerization. Nature Chem 10, 1222–1228 (2018). https://doi.org/10.1038/s41557-018-0134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0134-4
This article is cited by
-
Photocatalytic conversion of sugars to 5-hydroxymethylfurfural using aluminium(III) and fulvic acid
Nature Communications (2023)
-
Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations
Nature Communications (2023)
-
One-Step Acid Bleachable Pretreatment with a Recyclable Acid Hydrotrope and Chlorate for Biomass Valorization
BioEnergy Research (2023)
-
Sustainable polyesters via direct functionalization of lignocellulosic sugars
Nature Chemistry (2022)
-
Chemical, physical and biological methods to convert lignocellulosic waste into value-added products. A review
Environmental Chemistry Letters (2022)