Abstract
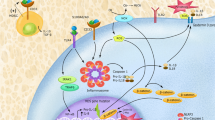
PTEN is a dual-specificity phosphatase that is frequently mutated in human cancer, and its deficiency in cancer has been associated with therapy resistance and poor survival. Although the intrinsic tumour-suppressor function of PTEN has been well established, evidence of its role in the tumour immune microenvironment is lacking. Here, we show that chemotherapy-induced antitumour immune responses and tumour suppression rely on myeloid-cell PTEN, which is essential for chemotherapy-induced activation of the NLRP3 inflammasome and antitumour immunity. PTEN directly interacts with and dephosphorylates NLRP3 to enable NLRP3–ASC interaction, inflammasome assembly and activation. Importantly, supplementation of IL-1β restores chemotherapy sensitivity in mouse myeloid cells with a PTEN deficiency. Clinically, chemotherapy-induced IL-1β production and antitumour immunity in patients with cancer is correlated with PTEN expression in myeloid cells, but not tumour cells. Our results demonstrate that myeloid PTEN can determine chemotherapy responsiveness by promoting NLRP3-dependent antitumour immunity and suggest that myeloid PTEN might be a potential biomarker to predict chemotherapy responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
MS data have been deposited in iProX with the primary accession code IPX0001867000. Source data are available online for Figs. 1–8 and Extended Data Figs. 1–8. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
References
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Zitvogel, L., Galluzzi, L., Smyth, M. J. & Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39, 74–88 (2013).
Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997).
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Worby, C. A. & Dixon, J. E. PTEN. Annu. Rev. Biochem. 83, 641–669 (2014).
Lee, Y. R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
George, S. et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 46, 197–204 (2017).
Peng, W. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Dillon, L. M. & Miller, T. W. Therapeutic targeting of cancers with loss of PTEN function. Curr. Drug Targets 15, 65–79 (2014).
Hildebrandt, M. A. et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J. Clin. Oncol. 27, 857–871 (2009).
Dave, B. et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J. Clin. Oncol. 29, 166–173 (2011).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Chen, L. & Guo, D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell. Mol. Immunol. 14, 581–589 (2017).
Munoz-Fontela, C., Mandinova, A., Aaronson, S. A. & Lee, S. W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 16, 741–750 (2016).
Di Cristofano, A. et al. Impaired Fas response and autoimmunity in Pten +/− mice. Science 285, 2122–2125 (1999).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Shrestha, S. et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 16, 178–187 (2015).
Li, S. et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat. Immunol. 17, 241–249 (2016).
Schabbauer, G. et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J. Immunol. 185, 468–476 (2010).
Sahin, E. et al. Loss of Phosphatase and tensin homolog in APCs impedes Th17-mediated autoimmune encephalomyelitis. J. Immunol. 195, 2560–2570 (2015).
Yue, S. et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. J. Immunol. 192, 5343–5353 (2014).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Broderick, L., De Nardo, D., Franklin, B. S., Hoffman, H. M. & Latz, E. The inflammasomes and autoinflammatory syndromes. Annu. Rev. Pathol. 10, 395–424 (2015).
Xiao, T. S. Innate immunity and inflammation. Cell. Mol. Immunol. 14, 1–3 (2017).
Kuttke, M. et al. Myeloid PTEN deficiency impairs tumor-immune surveillance via immune-checkpoint inhibition. Oncoimmunology 5, e1164918 (2016).
Aymeric, L. et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 70, 855–858 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Di Cristofano, A. & Pandolfi, P. P. The multiple roles of PTEN in tumor suppression. Cell 100, 387–390 (2000).
Myers, M. P. et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl Acad. Sci. USA 94, 9052–9057 (1997).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Lee, Y. R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
Weng, L. P., Brown, J. L. & Eng, C. PTEN coordinates G1 arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet 10, 599–604 (2001).
Myers, M. P. et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl Acad. Sci. USA 95, 13513–13518 (1998).
Papa, A. et al. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell 157, 595–610 (2014).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 (2016).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Raftopoulou, M., Etienne-Manneville, S., Self, A., Nicholls, S. & Hall, A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303, 1179–1181 (2004).
Shi, Y. et al. PTEN is a protein tyrosine phosphatase for IRS1. Nat. Struct. Mol. Biol. 21, 522–527 (2014).
Wang, L. et al. PTEN-L is a novel protein phosphatase for ubiquitin dephosphorylation to inhibit PINK1–Parkin-mediated mitophagy. Cell Res. 28, 787–802 (2018).
Wozniak, D. J. et al. PTEN is a protein phosphatase that targets active PTK6 and inhibits PTK6 oncogenic signaling in prostate cancer. Nat. Commun. 8, 1508 (2017).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606 (2018).
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Abderrazak, A. et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 4, 296–307 (2015).
Song, N. et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 68, 185–197 (2017).
Stutz, A. et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 214, 1725–1736 (2017).
Spalinger, M. R. et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Invest. 126, 1783–1800 (2016).
Gong, T., Jiang, W. & Zhou, R. Control of inflammasome activation by phosphorylation. Trends Biochem. Sci. 43, 685–699 (2018).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Ma, W. T. et al. Modulation of liver regeneration via myeloid PTEN deficiency. Cell Death Dis. 8, e2827 (2017).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015).
Gibby, K. et al. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan. Breast Cancer Res. 14, R67 (2012).
Han, D. et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274 (2019).
Wang, C. J. et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl. Immunohistochem. Mol. Morphol. 17, 530–535 (2009).
Yan, Y. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38, 1154–1163 (2013).
Huang, Y. et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10, e8689 (2018).
Wang, Y. et al. A proteomics landscape of circadian clock in mouse liver. Nat. Commun. 9, 1553 (2018).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Acknowledgements
We thank J. Tschopp and Z. Lian for providing mice lines. This research was supported by the National Key research and development program of China (grant number 2019YFA0508503), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB29030102), the National Natural Science Foundation of China (grant numbers 31770991, 91742202, 81525013, 81722022, 81788101, 81821001 and 81722037), the Young Talent Support Program and Fundamental Research Funds for the Central Universities and the University Synergy Innovation Program of Anhui Province (GXXT-2019-026).
Author information
Authors and Affiliations
Contributions
Y. Huang, H.W., Y. Hao and H. Lin performed most of the experiments of this work. M.D., B.S., Y.J. and H. Li collected patient samples. L.S. and Y.W. performed the MS analyses. J.Y. performed the protein purification. Q.L., Z.S., G.K., H.Z., L.B., T.J., C.W., Y.M., Y.C., C.D., S.L., Y.P., W.J. and R.Z. designed the research. Y. Huang, W.J. and R.Z. wrote the manuscript. W.J. and R.Z. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Myeloid-cell PTEN is necessary for chemotherapy.
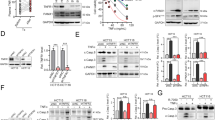
a, Immunoblot analysis of PTEN expression in purified tumour-infiltrating macrophages (TIMs). n = 2 biologically independent experiments. b, Immunoblot analysis of PTEN expression in purified peritoneal macrophages. n = 3 biologically independent experiments. c, 5 × 105 MCA205 cells were implanted sub-cutaneously into Ptenf/f or PtenmKO mice. Mitoxantrone were injected intraperitoneally when the size of tumor reached 20-45 mm2. Images of tumors at day 21 after tumor cells implanted. n = 10 biologically independent animals. d-g, 8-week old Ptenf/f or PtenmKO mice were inoculated with 5 × 105 MCA205, TC-1 or 3 × 105 EL-4 tumor cells sub-cutaneously or 3 × 105 PY8119 tumor cells at fourth mammary fat pads. When the size of tumor reached 20–45 mm2 of TC-1 (e), MCA205 (f) and Py8119 (g), or 70-90 mm2 of EL-4 (d), Mitoxantrone (MTX, 5.17 mg/kg), oxaliplatin (OXP, 5 mg/kg) or epirubicin (EPI, 7mg/kg) were injected intraperitoneally as indicated. Tumor size at various times (horizontal axis) after tumor cells injection. n = 5 biologically independent animals. h, i, FACS analysis of CD44 and CD62L expression in CD8+ T cells from tumor tissues after 5-day chemotherapy. n = 5 biologically independent animals. d-g, i, Data are shown as mean ± s.e.m. Statistics were analyzed using two-sided unpaired Student’s t-test. Source data are provided in Source Data Extended Data Fig. 1.
Extended Data Fig. 2 The tumoricidal activity of mitoxantrone depends on the NLRP3 inflammasome.
a, Immunoblot analysis of NLRP3 expression in purified tumour-infiltrating macrophages (TIMs). n = 2 biologically independent experiments. b, Immunoblot analysis of NLRP3 expression in purified peritoneal macrophages. n = 3 biologically independent experiments. c-f, 5 × 105 MCA205 cells were implanted sub-cutaneously into Nlrp3+/+ or Nlrp3−/− mice. When the size of tumor reached 20-45 mm2, Mitoxantrone were injected intraperitoneally. Survival analysis of Nlrp3+/+ and Nlrp3−/− mice at various times (horizontal axis) after tumor cells injection (c; n = 7 biologically independent animals). Images of tumors at day 21 after tumor cells implanted (d; n = 8 biologically independent animals). Tumor size at various times (horizontal axis) after tumor cells injection (e; n = 8 biologically independent animals). ELISA analysis of IFN-γ secretion of inguinal lymph node cells after 5-day chemotherapy (f; n = 7 biologically independent animals). e, f, Data are shown as mean ± s.e.m. Statistics were analyzed using two-sided log-rank (Mantel–Cox) test (c) or two-sided unpaired Student’s t-test (e, f). Source data are provided in Source Data Extended Data Fig. 2.
Extended Data Fig. 3 PTEN is required for NLRP3 inflammasome activation.
a, b, PTEN-knockdown THP-1 cells were primed with 200 ng/ml LPS for 3 h and then stimulated with various doses (above lanes) nigericin. Immunoblot analysis of IL-1β and caspase-1 in culture supernatants, and immunoblot analysis of the expression of PTEN, pro-IL-1β and pro-caspase-1 in lysates of those cells (a; n = 2 biologically independent experiments). ELISA analysis of IL-1β in culture supernatants (b; n = 4 biologically independent experiments). c, d, ELISA analysis of TNF-α (c) or IL-6 (d) in culture supernatants of Ptenf/f or PtenmKO BMDMs primed with LPS for 3 h and then stimulated with various doses (above lanes) nigericin. n = 4 biologically independent experiments. e, g, Immunoblot analysis of IL-1β and caspase-1 in culture supernatants of Ptenf/f or PtenmKO BMDMs primed with 50 ng/ml LPS for 3 h and then stimulated with poly A:T (e) or salmonella (g), and immunoblot analysis of pro-IL-1β and pro-caspase-1 in lysates of those cells. n = 2 biologically independent experiments. f, h, ELISA analysis of IL-1β in culture supernatants of Ptenf/f or PtenmKO BMDMs primed with 50 ng/ml LPS for 3 h and then stimulated with poly A:T (f) or salmonella (h). n = 4 biologically independent experiments. i, Immunoblot analysis of the expression of p-AKT, total AKT and PTEN in lysates of those cells of Ptenf/f or PtenmKO BMDMs primed with LPS for 3 h, and then treated with wortmannin (1 µM) or LY294002 (5 µM) for 30 min. n = 2 biologically independent experiments. b-d, f, h, Data are shown as mean ± s.e.m. Statistics were analyzed using two-sided unpaired Student’s t-test. Source data are provided in Source Data Extended Data Fig. 3.
Extended Data Fig. 4 PTEN has no effects on potassium efflux or mitochondrial damage during NLRP3 activation.
a, Qualification analysis of potassium efflux in Ptenf/f or PtenmKO BMDMs primed with LPS for 3 h, and then stimulated with nigericin for 30 min. Mock groups n = 2 biologically independent experiments, treated groups n = 4 biologically independent experiments. b, Confocal microscopy analysis in Ptenf/f or PtenmKO BMDMs primed with LPS for 3 h, and then stimulated with nigericin for 30 min, followed by staining with Mitosox, Mitotracker red and DAPI. Scale bars, 10 μm. n = 2 biologically independent experiments. c, Commassie blue stainning of recombinant human NLRP3 and PTEN protein. d, e, Scheme of NLRP3 (d) or PTEN (e) domain structure. a, Data are shown mean ± s.e.m. Statistics were analyzed using two-sided unpaired Student’s t-test. Source data are provided in Source Data Extended Data Fig. 4.
Extended Data Fig. 5 PTEN mediates NLRP3 dephosphorylation at Y32 is required for inflammasome activation.
a, Extracted ion chromatograms (XIC) of the peptide phosphorylated at Tyr32 (MHLEDpYPPQKGCIPLPR), Thr193 (pTKTCESPVSPIK) and Thr 195 (pTCESPVSPIK) of NLRP3 with or without PTEN overexpression. b, Multiple sequence alignment of NLRP3 from the indicated species. c, d, Immunoblot analysis of IL-1β and caspase-1 (c; n = 2 biologically independent experiments.) or ELISA analysis of IL-1β (d; n = 4 biologically independent experiments) in culture supernatants of Ptenf/fNlrp3−/− or PtenmKONlrp3−/− BMDMs transduced with mouse WT or mutant pLEX-NLRP3 lentivirus, and then primed with LPS, stimulated with nigericin. e, Immunoblot analysis of the immunoprecipitated Flag-tagged WT or nonphosphorylatable mutants (Y30A) NLRP3 with mouse NLRP3 specific phospho-Tyr30 antibody (p-NLRP3). n = 2 biologically independent experiments. f, Immunoblot analysis of the immunoprecipitated Flag-tagged NLRP3 with p-NLRP3 antibody when NLRP3 were coexpressed with PTEN. n = 2 biologically independent experiments. g, Commassie blue stainning of recombinant mouse NLRP3 and PTEN, PTEN mutant C124S, G129E or G129R protein. d, Data are shown as mean ± s.e.m. Statistics were analyzed using two-sided unpaired Student’s t-test. Source data are provided in Source Data Extended Data Fig. 5.
Extended Data Fig. 6 Construction of Nlrp3Y30E/Y30E mice.
a, Nlrp3Y30E/Y30E mice was generated using CRISPR/Cas9-mediated genome editing. The sequence of guide RNA (gRNA) binding site on the homologous DNA sequence had indicated. b, Nlrp3Y30E/Y30E mice was validated by DNA sequencing.
Extended Data Fig. 7 Myeloid PTEN determines chemotherapy-induced antitumor immunity through activating NLRP3 inflammasome.
a–c, 5 × 105 MCA205 cells were implanted sub-cutaneously into Nlrp3+/+ or Nlrp3Y30E/Y30E mice. Mitoxantrone were injected intraperitoneally when the size of tumor reached 20-45 mm2. Images of tumors at day 21 after tumor cells implanted (a; n = 6 biologically independent animals). FACS analysis of intracellular IFN-γ in CD8+ T cells from inguinal lymph nodes after 5-day chemotherapy (b, c; n = 4 biologically independent animals). d-f, 5 × 105 MCA205 cells were implanted sub-cutaneously into Ptenf/fNlrp3+/+, PtenmKONlrp3+/+, Ptenf/fNlrp3−/− or PtenmKONlrp3−/− mice. Mitoxantrone were injected intraperitoneally when the size of tumor reached 20-45 mm2. Images of tumors at day 21 after tumor cells implanted (d; n = 5 biologically independent animals). FACS analysis of intracellular IFN-γ in CD8+ T cells from inguinal lymph nodes after 5-day chemotherapy (e, f; n = 4 biologically independent animals). c, f, Data are shown as mean ± s.e.m. Statistics were analyzed using two-sided unpaired Student’s t-test. Source data are provided in Source Data Extended Data Fig. 7.
Extended Data Fig. 8 Myeloid PTEN correlates chemotherapy-induced antitumor immunity in human cancer patients.
a, Kaplan–Meier curve analysis of the overall survival probabilities of patients with breast cancer influenced by PTEN expression. Ptenlow groups n = 177 biologically independent patients, Ptenhigh groups n = 449 biologically independent patients. b-d, Correlations analysis between the MOD of PTEN in tumor cells with IL-1β concentrations in serum (b), CD8α (c) or IFNG (d) mRNA expression in tumor tissues from cohort 1. n = 32 biologically independent patients. e, Correlations analysis between the MOD of PTEN in tumor cells and IL-1β concentrations in serum from cohort 2. n = 34 biologically independent patients. Statistics were analyzed using two-sided log-rank (Mantel–Cox) test (a) or two-sided unpaired Student’s t-test (b-e). Source data are provided in Source Data Extended Data Fig. 8.
Supplementary information
Supplementary Information
Supplementary Fig. 1
Supplementary Tables 1–4
Supplementary Table 1: phosphorylation sites analyses of NLRP3 by MS. Supplementary Table 2: MS identification the change of NLRP3 phosphorylation with or without PTEN overexpression. Supplementary Table 3: summary of information about the patients with breast cancer who have been treated with AAC (cohort 1). Supplementary Table 4: summary of information about patients with breast cancer before they were treated with AAC (cohort 2).
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Huang, Y., Wang, H., Hao, Y. et al. Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nat Cell Biol 22, 716–727 (2020). https://doi.org/10.1038/s41556-020-0510-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-0510-3
This article is cited by
-
HECTD3 inhibits NLRP3 inflammasome assembly and activation by blocking NLRP3-NEK7 interaction
Cell Death & Disease (2024)
-
Cold atmospheric plasma stabilizes mismatch repair for effective, uniform treatment of diverse colorectal cancer cell types
Scientific Reports (2024)
-
Divergent functions of NLRP3 inflammasomes in cancer: a review
Cell Communication and Signaling (2023)
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Exosomal PD-L1 confers chemoresistance and promotes tumorigenic properties in esophageal cancer cells via upregulating STAT3/miR-21
Gene Therapy (2023)