Abstract
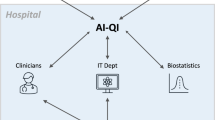
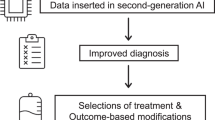
The complex relationships between continuously monitored health signals and therapeutic regimens can be modelled via machine learning. However, the clinical implementation of the models will require changes to clinical workflows. Here we outline ClinAIOps (‘clinical artificial-intelligence operations’), a framework that integrates continuous therapeutic monitoring and the development of artificial intelligence (AI) for clinical care. ClinAIOps leverages three feedback loops to enable the patient to make treatment adjustments using AI outputs, the clinician to oversee patient progress with AI assistance, and the AI developer to receive continuous feedback from both the patient and the clinician. We lay out the central challenges and opportunities in the deployment of ClinAIOps by means of examples of its application in the management of blood pressure, diabetes and Parkinson’s disease. By enabling more frequent and accurate measurements of a patient’s health and more timely adjustments to their treatment, ClinAIOps may substantially improve patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tison, G. H. et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 3, 409–416 (2018).
Dörr, M. et al. The WATCH AF Trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin. Electrophysiol. 5, 199–208 (2019).
Galindo, R. J. et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J. Diabetes Sci. Technol. 14, 1035–1064 (2020).
Johnston, L., Wang, G., Hu, K., Qian, C. & Liu, G. Advances in biosensors for continuous glucose monitoring towards wearables. Front. Bioeng. Biotechnol. 9, 733810 (2021).
Martens, T. et al. Effect of continuous glucose monitoring on glycemic control in patients with Type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 325, 2262–2272 (2021).
Tschider, C. A. Medical device artificial intelligence: the new tort frontier. BYU Law Rev. 46, 1551 (2020).
Simon, D. A., Shachar, C. & Glenn Cohen, I. Unsettled liability issues for ‘prediagnostic’ wearables and health-related products. JAMA 328, 1391–1392 (2022).
Benroubi, M. Fear, guilt feelings and misconceptions: barriers to effective insulin treatment in type 2 diabetes. Diabetes Res. Clin. Pract. 93, S97–S99 (2011).
Moor, M. et al. Foundation models for generalist medical artificial intelligence. Nature 616, 259–265 (2023).
Berwick, D. M., Nolan, T. W. & Whittington, J. The triple aim: care, health and cost. Health Aff. 27, 759–769 (2008).
Assadi, A. et al. An integration engineering framework for machine learning in healthcare. Front. Digit. Health 4, 932411 (2022).
Breaux-Shropshire, T. L., Judd, E., Vucovich, L. A., Shropshire, T. S. & Singh, S. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr. Blood Press. Control 8, 43–49 (2015).
Siontis, K. C., Noseworthy, P. A., Attia, Z. I. & Friedman, P. A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 18, 465–478 (2021).
Attia, Z. I. et al. Novel bloodless potassium determination using a signal‐processed single‐lead ECG. J. Am. Heart Assoc. 5, e002746 (2016).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 4, 2196–2204 (2019).
Powers, R. et al. Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci. Transl. Med. 13, eabd7865 (2021).
Fletcher, R. R., Tam, S., Omojola, O., Redemske, R. & Kwan, J. Wearable sensor platform and mobile application for use in cognitive behavioral therapy for drug addiction and PTSD. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 1802–1805 (2011).
Russell-Jones, D., Pouwer, F. & Khunti, K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes. Metab. 20, 488–496 (2018).
Przezak, A., Bielka, W. & Molęda, P. Fear of hypoglycemia—an underestimated problem. Brain Behav. 12, e2633 (2022).
Yeh, T., Yeung, M. & Mendelsohn Curanaj, F. A. Managing patients with insulin pumps and continuous glucose monitors in the hospital: to wear or not to wear. Curr. Diab. Rep. 21, 7 (2021).
Waks, J. W. et al. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: results from the Tailored Anticoagulation for Non-Continuous Atrial Fibrillation (TACTIC-AF) pilot study. Heart Rhythm 15, 1601–1607 (2018).
Passman, R. et al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the rhythm evaluation for anticoagulation with continuous monitoring (React.com) pilot study. J. Cardiovasc. Electrophysiol 27, 264–270 (2016).
Wasserlauf, J. et al. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 12, e006834 (2019).
Leading science, research and technology leaders join forces to accelerate REACT-AF trial. American Heart Association (29 August 2022).
Bienefeld, N. et al. Solving the explainable AI conundrum by bridging clinicians’ needs and developers’ goals. NPJ Digit. Med. 6, 94 (2023).
Attig, C. & Franke, T. Abandonment of personal quantification: a review and empirical study investigating reasons for wearable activity tracking attrition. Comput. Hum. Behav. 102, 223–237 (2020).
Wang, T. et al. Identifying major impact factors affecting the continuance intention of mHealth: a systematic review and multi-subgroup meta-analysis. NPJ Digit. Med. 5, 145 (2022).
Meyerowitz-Katz, G. et al. Rates of attrition and dropout in app-based interventions for chronic disease: systematic review and meta-analysis. J. Med. Internet Res. 22, e20283 (2020).
Helander, E., Kaipainen, K., Korhonen, I. & Wansink, B. Factors related to sustained use of a free mobile app for dietary self-monitoring with photography and peer feedback: retrospective cohort study. J. Med. Internet Res. 16, e109 (2014).
Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 14, eabn6036 (2022).
Zhang, Y., Suda, N., Lai, L. & Chandra, V. Hello Edge: keyword spotting on microcontrollers. Preprint at https://arxiv.org/abs/1711.07128 (2017).
Basaklar, T., Tuncel, Y., An, S. & Ogras, U. Wearable devices and low-power design for smart health applications: challenges and opportunities. In Proc. 2021 IEEE/ACM International Symposium on Low Power Electronics and Design (ISLPED) (eds Li, H. & Augustine, C.) 1 (IEEE, 2021).
Sundrani, S. et al. Predicting patient decompensation from continuous physiologic monitoring in the emergency department. NPJ Digit. Med. 6, 60 (2023).
Jackson, C., Shahsahebi, M., Wedlake, T. & DuBard, C. A. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann. Fam. Med. 13, 115–122 (2015).
Erb, M. K. et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinsonas disease.npj Digit. Med. 3, 6 (2020).
Steinkirchner, A. B. et al. Self-report of chronic diseases in old-aged individuals: extent of agreement with general practitioner medical records in the German AugUR study. J. Epidemiol. Community Health 76, 931–938 (2022).
Pirtošek, Z. et al. Update on the management of Parkinson’s disease for general neurologists. Parkinsonas Dis 2020, 9131474 (2020).
Shalash, A., Spindler, M. & Cubo, E. Global perspective on telemedicine for Parkinson’s disease. J. Parkinsons Dis. 11, S11–S18 (2021).
Greenland, J. C., Williams-Gray, C. H. & Barker, R. A. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur. J. Neurosci. 49, 328–338 (2019).
Drew, B. J. et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS ONE 9, e110274 (2014).
Chromik, J. et al. Computational approaches to alleviate alarm fatigue in intensive care medicine: a systematic literature review. Front. Digit. Health 4, 843747 (2022).
Nimri, R. et al. Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat. Med. 26, 1380–1384 (2020).
Zarrinpar, A. et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci. Transl. Med. 8, 333ra49 (2016).
Pantuck, A. J. et al. Modulating BET bromodomain inhibitor ZEN-3694 and enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Adv. Ther. 1, 1800104 (2018).
Kee, T. et al. Harnessing CURATE.AI as a digital therapeutics platform by identifying N-of-1 learning trajectory profiles. Adv. Ther. 2, 1900023 (2019).
Liang, W. et al. Advances, challenges and opportunities in creating data for trustworthy AI. Nat. Mach. Intell. 4, 669–677 (2022).
Zhang, A., Xing, L., Zou, J. & Wu, J. C. Shifting machine learning for healthcare from development to deployment and from models to data. Nat. Biomed. Eng. 6, 1330–1345 (2022).
Feng, J. et al. Clinical artificial intelligence quality improvement: towards continual monitoring and updating of AI algorithms in healthcare. NPJ Digit. Med. 5, 66 (2022).
Tang, J. et al. Corrigendum: application of machine-learning models to predict tacrolimus stable dose in renal transplant recipients. Sci. Rep. 8, 46936 (2018).
Liu, R., Li, X., Zhang, W. & Zhou, H.-H. Comparison of nine statistical model based warfarin pharmacogenetic dosing algorithms using the racially diverse International Warfarin Pharmacogenetic Consortium cohort database. PLoS ONE 10, e0135784 (2015).
Zhu, X. et al. A machine learning approach to personalized dose adjustment of lamotrigine using noninvasive clinical parameters. Sci. Rep. 11, 5568 (2021).
Jovanović, M. et al. Application of counter-propagation artificial neural networks in prediction of topiramate concentration in patients with epilepsy. J. Pharm. Pharm. Sci. 18, 856–862 (2015).
Tsichlaki, S., Koumakis, L. & Tsiknakis, M. Type 1 diabetes hypoglycemia prediction algorithms: systematic review. JMIR Diabetes 7, e34699 (2022).
US Food and Drug Administration et al. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-based Software as a Medical Device (SaMD) (US FDA, 019).
Salama, K., Kazmierczak, J. & Schut, D. Practitioners Guide to MLOps: A Framework for Continuous Delivery and Automation of Machine Learning (Google, 2021).
Utsumil, Y., Rudovicl, O. O., Petersonl, K., Guerrero, R. & Picardl, R. W. Personalized Gaussian processes for forecasting of Alzheimer’s Disease Assessment Scale-Cognition sub-scale (ADAS-Cog13). Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 4007–4011 (2018).
Liu, K. et al. Development and validation of a personalized model with transfer learning for acute kidney injury risk estimation using electronic health records. JAMA Netw. Open 5, e2219776 (2022).
Hard, A. et al. Federated learning for mobile keyboard prediction. Preprint at https://arxiv.org/abs/1811.03604 (2018).
Agarwal, R. Rehospitalization rates in hypertensive emergency. Hypertension 73, 49–51 (2019).
Miller, J., McNaughton, C., Joyce, K., Binz, S. & Levy, P. Hypertension management in emergency departments. Am. J. Hypertens. 33, 927–934 (2020).
Block, R. C. et al. Conventional pulse transit times as markers of blood pressure changes in humans. Sci. Rep. 10, 16373 (2020).
Nabeel, P. M., Jayaraj, J. & Mohanasankar, S. Single-source PPG-based local pulse wave velocity measurement: a potential cuffless blood pressure estimation technique. Physiol. Meas. 38, 2122–2140 (2017).
Islam, S. M. S. et al. Wearable cuffless blood pressure monitoring devices: a systematic review and meta-analysis. Eur. Heart J. Digit. Health 3, 323–337 (2022).
Zheng, Y.-L., Yan, B. P., Zhang, Y.-T. & Poon, C. C. Y. An armband wearable device for overnight and cuff-less blood pressure measurement. IEEE Trans. Biomed. Eng. 61, 2179–2186 (2014).
AI for Anti-Hypertensive Medication Titration (NIH NCBI, accessed 1 October 2023); https://clinicaltrials.gov/ct2/show/NCT05376683
Morawski, K. et al. Association of a Smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern. Med. 178, 802–809 (2018).
Baumann, B. M. et al. Provider self-report and practice: reassessment and referral of emergency department patients with elevated blood pressure. Am. J. Hypertens. 22, 604–610 (2009).
National Academies of Sciences, Engineering, and Medicine, Policy and Global Affairs, Committee on Women in Science, Engineering, and Medicine & Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups (National Academies Press, 2022).
Hoel, A. W. et al. Under-representation of women and ethnic minorities in vascular surgery randomized controlled trials. J. Vasc. Surg. 50, 349–354 (2009).
Adam, G. et al. Machine learning approaches to drug response prediction: challenges and recent progress. NPJ Precis. Oncol. 4, 19 (2020).
Goyal, M., Ospel, J. M., Kappelhof, M. & Ganesh, A. Challenges of outcome prediction for acute stroke treatment decisions. Stroke 52, 1921–1928 (2021).
Okumura, K. et al. Comparing patient and physician risk tolerance for bleeding events associated with anticoagulants in atrial fibrillation-evidence from the United States and Japan. Value in Health Regional Issues 6, 65–72 (2015).
Wiktorski, T. Challenges in causal inference from personal monitoring devices. In Proc. Federated Conference on Computer Science and Information Systems (ed. Jassem, K.) 99–102 (PTI, 2018).
Causality in digital medicine. Nat. Commun. 12, 5471 (2021).
Nogueira, A. R., Gama, J. & Ferreira, C. A. Improving prediction with causal probabilistic variables. In Proc. Advances in Intelligent Data Analysis XVIII (eds Berthold, M., Feelders, A. & Krempl, G.) 379–390 (Springer, 2020).
Deng, C., Ji, X., Rainey, C., Zhang, J. & Lu, W. Integrating machine learning with human knowledge. iScience 23, 101656 (2020).
Taylor, L. et al. Using virtual representations in mHealth application interventions for health-related behaviour change: a systematic review. Cogent Psychol. 9, 2069906 (2022).
El-Gayar, O., Ofori, M. & Nawar, N. On the efficacy of behavior change techniques in mHealth for self-management of diabetes: a meta-analysis. J. Biomed. Inform. 119, 103839 (2021).
Jakob, R. et al. Factors influencing adherence to mHealth apps for prevention or management of noncommunicable diseases: systematic review. J. Med. Internet Res. 24, e35371 (2022).
Everett, E. M. et al. A longitudinal view of disparities in insulin pump use among youth with type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Technol. Ther. 25, 131–139 (2023).
Davis, K. & Guterman, S. Rewarding excellence and efficiency in Medicare payments. Milbank Q. 85, 449–468 (2007).
Crowson, M. G. & Chan, T. C. Y. Machine learning as a catalyst for value-based health care. J. Med. Syst. 44, 139 (2020).
Acknowledgements
The authors thank A. Karargyris for helpful feedback.
Author information
Authors and Affiliations
Contributions
All authors discussed the content, reviewed and edited the manuscript, and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks David Bates, Randall Moorman and Xiangrong Liu for their contributions to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, E., Prakash, S., Janapa Reddi, V. et al. A framework for integrating artificial intelligence for clinical care with continuous therapeutic monitoring. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01115-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01115-0