Abstract
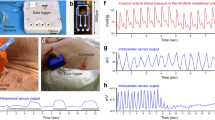
Devices for monitoring blood haemodynamics can guide the perioperative management of patients with cardiovascular disease. Current technologies for this purpose are constrained by wired connections to external electronics, and wireless alternatives are restricted to monitoring of either blood pressure or blood flow. Here we report the design aspects and performance parameters of an integrated wireless sensor capable of implantation in the heart or in a blood vessel for simultaneous measurements of pressure, flow rate and temperature in real time. The sensor is controlled via long-range communication through a subcutaneously implanted and wirelessly powered Bluetooth Low Energy system-on-a-chip. The device can be delivered via a minimally invasive transcatheter procedure or it can be mounted on a passive medical device such as a stent, as we show for the case of the pulmonary artery in a pig model and the aorta and left ventricle in a sheep model, where the device performs comparably to clinical tools for monitoring of blood flow and pressure. Battery-less and wireless devices such as these that integrate capabilities for flow, pressure and temperature sensing offer the potential for continuous monitoring of blood haemodynamics in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for Figs. 2–6 are provided with this paper. The raw and analysed datasets generated during the studies are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Custom-developed firmware for BLE SoCs and Android applications (UIs) for use on smartphones are available from the corresponding author on reasonable request. All requests for source code will be reviewed by the corresponding author to verify whether the request is subject to any intellectual property or confidentiality obligations.
References
Abbafati, C. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Mensah, G. A., Roth, G. A. & Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 74, 2529–2532 (2019).
Vincent, J. L. et al. Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit. Care 19, 224 (2015).
Kamel, H., Hegde, M., Johnson, D. R., Gage, B. F. & Johnston, S. C. Cost-effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke 41, 1514–1520 (2010).
Vennemann, B., Obrist, D. & Ro, T. A smartphone-enabled wireless and batteryless implantable blood flow sensor for remote monitoring of prosthetic heart valve function. PLoS ONE 15, e0227372 (2020).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Hu, S. et al. A wireless passive extra-arterial implantable blood pressure monitoring sensing system for rats. Microsyst. Technol. 27, 2595–2603 (2021).
Murphy, O. H. et al. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed. Microdevices 15, 737–749 (2013).
Laver, R. D., Wiersema, U. F. & Bersten, A. D. Echocardiographic estimation of mean pulmonary artery pressure in critically ill patients. Crit. Ultrasound J. 6, 9 (2014).
Garcia, J. et al. Distribution of blood flow velocity in the normal aorta: effect of age and gender. HHS Public Access 47, 487–498 (2019).
Sharman, J. E., Smart, N. A., Coombes, J. S. & Stowasser, M. Exercise and sport science australia position stand update on exercise and hypertension. J. Hum. Hypertens. 33, 837–843 (2019).
Bordes, S., Cichowski, E. & Homan, T. Physiology, Pulse Pressure (StatPearls, 2020).
Mebazaa, A., Karpati, P., Renaud, E. & Algotsson, L. Acute right ventricular failure—from pathophysiology to new treatments. Intensive Care Med. 30, 185–196 (2004).
Hadinnapola, C., Li, Q., Su, L., Pepke-Zaba, J. & Toshner, M. The resistance–compliance product of the pulmonary circulation varies in health and pulmonary vascular disease. Physiol. Rep. 3, e12363 (2015).
Kwon, K. et al. Miniaturized, light-adaptive, wireless dosimeters autonomously monitor exposure to electromagnetic radiation. Sci. Adv. 5, eaay2462 (2019).
Hearse, D. J. & Sutherland, F. J. Experimental models for the study of cardiovascular function and disease defining the question and identifying the model. Pharmacol. Res. 41, 597–603 (2000).
Lukács, E. et al. Overview of large animal myocardial infarction models (review). Acta Physiol. Hung. 99, 365–381 (2012).
Milani-Nejad, N. & Janssen, P. M. L. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014).
Camacho, P., Fan, H., Liu, Z. & He, J. Q. Small mammalian animal models of heart disease. Am. J. Cardiovasc. Dis. 6, 70–80 (2016).
Argueta, E. E. & Paniagua, D. Thermodilution cardiac output: a concept over 250 years in the making. Cardiol. Rev. 27, 138–144 (2019).
Stevens, J. H., Raffin, T. A., Mihm, F. G., Rosenthal, M. H. & Stetz, C. W. Thermodilution cardiac output measurement: effects of the respiratory cycle on its reproducibility. JAMA 253, 2240–2242 (1985).
Rajani, R., Hancock, J. & Chambers, J. B. The art of assessing aortic stenosis. Heart 98, 14–22 (2012).
Won, S. M. et al. Multimodal sensing with a three-dimensional piezoresistive structure. ACS Nano 13, 10972–10979 (2019).
Kim, H.-S. et al. Self-assembled nanodielectrics and silicon nanomembranes for low voltage, flexible transistors, and logic gates on plastic substrates. Appl. Phys. Lett. 95, 183504 (2009).
Acknowledgements
K.K. acknowledges support by the National Research Foundation (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning; no. 2021R1F1A106387111, no. 2022R1C1C1010555 and no. 2020R1A5A8018367). J.U.K. and T.K. were supported by the NRF funded by the Korean government (MSIT; NRF-2019M3C7A1032076 and NRF-2020M3H4A1A03082897). S.M.W. acknowledges support by the NRF grant funded by the Korea government (MSIP, ICT & Future Planning; no. NRF-2021R1C1C1009410 and no. IITP-2020-0-01821). R.A. acknowledges support from the National Science Foundation Graduate Research Fellowship (NSF grant number DGE-1842165) and Ford Foundation Predoctoral Fellowship. J.A.R. acknowledges support from the National Institute on Aging of the National Institutes of Health (NIH R43AG067835). We acknowledge funding from Wearifi Inc., and the Querrey-Simpson Institute for Bioelectronics at Northwestern University for support of this work. This work made use of the NUFAB facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the MRSEC programme (NSF DMR-1720139) at the Materials Research Center, the International Institute for Nanotechnology (IIN), the Keck Foundation and the State of Illinois, through the IIN.
Author information
Authors and Affiliations
Contributions
K.K. and J.A.R. conceived the idea and designed the research. K.K., J.U.K. and J.A.R. analysed data and wrote the manuscript. K.K. designed the hardware for the wireless electronic system. K.K. and K.S.C. performed software design and software validation. J.U.K. and S.M.W. performed and were involved in the manufacturing of the sensor modules. J.Z., R.A., H.W. and Y.H. performed mechanical modelling. K.K. and J.U.K. performed research and led the experimental works with support from H.J., K.H.L., J.-H.K., S. Y., Y.J.K., J.K., J.L., Y.P., W.L., T.K. and A.B.
Corresponding authors
Ethics declarations
Competing interests
A.B. and J.A.R. are co-founders of Hemorhythmics Inc., which has potential commercial interest in the technology described in this work. A.B. and J.A.R. are co-founders of the company. A.B is an employee of Wearifi Inc., which may wish to pursue commercialization of this technology in future. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jun Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, figures and references.
Supplementary Video 1
Movement of the sensing module inside the PA of porcine hearts recorded by a borescope.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwon, K., Kim, J.U., Won, S.M. et al. A battery-less wireless implant for the continuous monitoring of vascular pressure, flow rate and temperature. Nat. Biomed. Eng 7, 1215–1228 (2023). https://doi.org/10.1038/s41551-023-01022-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01022-4
This article is cited by
-
A Skin-Inspired Self-Adaptive System for Temperature Control During Dynamic Wound Healing
Nano-Micro Letters (2024)