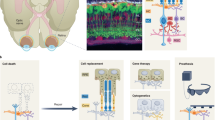
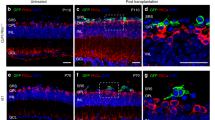
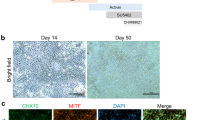
Abstract
Late-stage retinal degenerative disease involving photoreceptor loss can be treated by optogenetic therapy, cell transplantation and retinal prostheses. These approaches aim to restore light sensitivity to the retina as well as visual perception by integrating neuronal responses for transmission to the cortex. In age-related macular degeneration, some cell-based therapies also aim to restore photoreceptor-supporting tissue to prevent complete photoreceptor loss. In the earlier stages of degeneration, gene-replacement therapy could attenuate retinal-disease progression and reverse loss of function. And gene-editing strategies aim to correct the underlying genetic defects. In this Review, we highlight the most promising gene therapies, cell therapies and retinal prostheses for the treatment of retinal disease, discuss the benefits and drawbacks of each treatment strategy and the factors influencing whether functional tissue is reconstructed and repaired or replaced with an electronic device, and summarize upcoming technologies for enhancing the restoration of vision.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sohocki, M. M. et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum. Mutat. 17, 42–51 (2001).
Liew, G., Michaelides, M. & Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 4, e004015 (2014).
Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health 2, e106–e116 (2014).
Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017).
Xue, K. et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 24, 1507–1512 (2018).
Cukras, C. et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol. Ther. 26, 2282–2294 (2018).
Cehajic-Kapetanovic, J. et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 26, 354–359 (2020).
Kantor, A. et al. CRISPR genome engineering for retinal diseases. Prog. Mol. Biol. Transl. Sci. 182, 29–79 (2021).
Quinn, J. et al. Genome-editing strategies for treating human retinal degenerations. Hum. Gene Ther. 32, 247–259 (2021).
Maeder, M. L. et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 25, 229–233 (2019).
Jonas, J. B. et al. Glaucoma. Lancet 390, 2183–2193 (2017).
Guy, J. et al. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results. Ophthalmology 124, 1621–1634 (2017).
Vignal, C. et al. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy. Ophthalmology 125, 945–947 (2018).
Wu, S., Chang, K. C., Nahmou, M. & Goldberg, J. L. Induced pluripotent stem cells promote retinal ganglion cell survival after transplant. Invest. Ophthalmol. Vis. Sci. 59, 1571–1576 (2018).
Zhang, X. et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor. Neurol. Neurosci. 38, 131–140 (2020).
Suen, H. C. et al. Transplantation of retinal ganglion cells derived from male germline stem cell as a potential treatment to glaucoma. Stem Cells Dev. 28, 1365–1375 (2019).
Jones, B. W. et al. Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 464, 1–16 (2003).
Marc, R. E. & Jones, B. W. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol. 28, 139–147 (2003).
Marc, R. E., Jones, B. W., Watt, C. B. & Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 22, 607–655 (2003).
Jones, B. W. & Marc, R. E. Retinal remodeling during retinal degeneration. Exp. Eye Res. 81, 123–137 (2005).
Marc, R. E. et al. Neural reprogramming in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 48, 3364–3371 (2007).
Cuenca, N. et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 43, 17–75 (2014).
Jones, B. W. et al. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 150, 149–165 (2016).
Krishnamoorthy, V. et al. Retinal remodeling: concerns, emerging remedies and future prospects. Front. Cell Neurosci. 10, 38 (2016).
Pfeiffer, R. L., Marc, R. E. & Jones, B. W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 74, 100771 (2019).
Eleftheriou, C. G. et al. Meclofenamic acid improves the signal to noise ratio for visual responses produced by ectopic expression of human rod opsin. Mol. Vis. 23, 334–345 (2017).
Telias, M. et al. Retinoic acid induces hyperactivity, and blocking its receptor unmasks light responses and augments vision in retinal degeneration. Neuron 102, 574–586.e5 (2019).
Acland, G. M. et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28, 92–95 (2001).
Bainbridge, J. W. B. et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 (2008).
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
Cideciyan, A. V. et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl Acad. Sci. USA 105, 15112–15117 (2008).
Fischer, M. D. et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: a nonrandomized controlled trial. JAMA Ophthalmol. 138, 643–651 (2020).
Josan, A. S. et al. Microperimetry Hill of vision and volumetric measures of retinal sensitivity. Transl. Vis. Sci. Technol. 10, 12 (2021).
MacDonald, I. M. et al. Perspectives on gene therapy: choroideremia represents a challenging model for the treatment of other inherited retinal degenerations. Trans. Vis. Sci. Tech. 9, 17 (2020).
McClements, M. E. et al. An AAV dual vector strategy ameliorates the Stargardt phenotype in adult Abca4-/- mice. Hum. Gene Ther. 30, 590–600 (2019).
Hirsch, M. L., Wolf, S. J. & Samulski, R. J. Delivering transgenic DNA exceeding the carrying capacity of AAV vectors. Methods Mol. Biol. 1382, 21–39 (2016).
Trapani, I. et al. Improved dual AAV vectors with reduced expression of truncated proteins are safe and effective in the retina of a mouse model of Stargardt disease. Hum. Mol. Genet. 24, 6811–6825 (2015).
T Trapani, I. et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 6, 194–211 (2014).
Yi-Ting, T. et al. Clustered regularly interspaced short palindromic repeats-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology 125, 1421–1430 (2018).
Diakatou, M., Manes, G., Bocquet, B., Meunier, I. & Kalatzis, V. Genome editing as a treatment for the most prevalent causative genes of autosomal dominant retinitis pigmentosa. Int. J. Mol. Sci. 20, 2542 (2019).
Sternberg, S. H., Redding, S., Jinek, M., Greene, E. C. & Doudna, J. A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507, 62–67 (2014).
Sander, J. D. & Joung, J. K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Cai, Y. et al. In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway. Sci. Adv. 5, eaav3335 (2019).
Bakondi, B. et al. In vivo CRISPR/Cas9 Gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol. Ther. 24, 556–563 (2016).
Tsai, Y. et al. CRISPR-based genome surgery for the treatment of autosomal dominant retinitis. Ophthalmology 125, 1421–1430 (2018).
McClements, M. E., Staurenghi, F., MacLaren, R. E. & Cehajic-Kapetanovic, J. Optogenetic gene therapy for the degenerate retina: recent advances. Front. Neurosci. 11, 570909 (2020).
Bi, A. et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33 (2006).
Zhang, Y., Ivanova, E., Bi, A. & Pan, Z. H. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J. Neurosci. 29, 9186–9196 (2009).
Thyagarajan, S. et al. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J. Neurosci. 30, 8745–8758 (2010).
Doroudchi, M. M. et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 19, 1220–1229 (2011).
Sengupta, A. et al. Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol. Med. 8, 1248–1264 (2016).
Lagali, P. S. et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 11, 667–675 (2008).
Macé, E. et al. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV restores ON and OFF visual responses in blind mice. Mol. Ther. 23, 7–16 (2015).
Busskamp, V. et al. Genetic rectivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417 (2010).
Gauvain, G. et al. Optogenetic therapy: high spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 4, 125 (2021).
Sahel, J. A. et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 27, 1223–1229 (2021).
Cehajic-Kapetanovic, J. et al. Restoration of vision with ectopic expression of human rod opsin. Curr. Biol. 25, 2111–2122 (2015).
Gaub, B. M., Berry, M. H., Holt, A. E., Isacoff, E. Y. & Flannery, J. G. Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol. Ther. 23, 1562–1571 (2015).
Lin, B., Koizumi, A., Tanaka, N., Panda, S. & Masland, R. H. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl Acad. Sci. USA 105, 16009–16014 (2008).
De Silva, S. R. et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc. Natl Acad. Sci. USA 114, 11211–11216 (2017).
van Wyk, M., Pielecka-Fortuna, J., Löwel, S. & Kleinlogel, S. Restoring the ON switch in blind retinas: opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 13, e1002143 (2015).
Berry, M. H. et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 10, 1221 (2019).
Gaub, B. M. et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl Acad. Sci. USA 111, E5574–E5583 (2014).
Berry, M. H. et al. Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 8, 1862 (2017).
Tochitsky, I. et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813 (2014).
Tochitsky, I., Trautman, J., Gallerani, N., Malis, J. G. & Kramer, R. H. Restoring visual function to the blind retina with a potent, safe and long-lasting photoswitch. Sci. Rep. 7, 45487 (2017).
Cehajic-Kapetanovic, J. et al. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol. Vis. 17, 1771–1783 (2011).
Cehajic-Kapetanovic, J., Milosavljevic, N., Bedford, R. A., Lucas, R. J. & Bishop, P. N. Efficacy and safety of glycosidic enzymes for improved gene delivery to the retina following intravitreal injection in mice. Mol. Ther. Methods Clin. Dev. 9, 192–202 (2017).
Fick, A. ‘On liquid diffusion’. Ann. Phys. Chem. 94, 59 (1855).
Dalkara, D. et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra76 (2013).
Kotterman, M. A. et al. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 22, 116–126 (2015).
Cehajic-Kapetanovic, J. et al. Highest reported visual acuity after electronic retinal implantation. Acta Ophthalmol. 98, 736–740 (2020).
McClements, M. E. et al. AAV induced expression of human rod and cone opsin in bipolar cells of a mouse model of retinal degeneration, BioMed. Res. Int. https://doi.org/10.1155/2021/4014797 (2021).
Thanos, C. G. et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 10, 1617–1622 (2004).
Lund, R. D. et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25, 602–611 (2007).
Ho, A. C. et al. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am. J. Ophthalmol. 179, 67–80 (2017).
Park, S. S. Cell therapy applications for retinal vascular diseases: diabetic retinopathy and retinal vein occlusion. Invest. Ophthalmol. Vis. Sci. 57, ORSFj1–ORSFj10 (2016).
Park, S. S. et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest. Ophthalmol. Vis. Sci. 56, 81–89 (2014).
Otani, A. et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Invest. 114, 765–774 (2004).
Koh, S. et al. Subretinal human umbilical tissue-derived cell transplantation preserves retinal synaptic connectivity and attenuates Müller glial reactivity. J. Neurosci. 38, 2923–2943 (2018).
Koh, S. et al. Human umbilical tissue-derived cells promote synapse formation and neurite outgrowth via thrombospondin family proteins. J. Neurosci. 35, 15649–15665 (2015).
Börger, V. et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 18, 1450 (2017).
Zhou, J. et al. Retinal progenitor cells release extracellular vesicles containing developmental transcription factors, microRNA and membrane proteins. Sci. Rep. 8, 2823 (2018).
Knickelbein, J. E. et al. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 57, 4101–4107 (2016).
Kashani, A. H. et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 10, eaao4097 (2018).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017).
da Cruz, L. et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 36, 328–337 (2018).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Schwartz, S. D., Tan, G., Hosseini, H. & Nagiel, A. Subretinal transplantation of embryonic stem cell–derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest. Ophthalmol. Vis. Sci. 57, ORSFc1–ORSFc9 (2016).
Sharma, R. et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 11, eaat5580 (2019).
Ben M’Barek, K. et al. Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med. 9, eaai7471 (2019).
Kuppermann, B. D., Boyer, D. S., Mills, B., Yang, J. & Klassen, H. J. Safety and activity of a single, intravitreal injection of human retinal progenitor cells (jCell) for treatment of retinitis pigmentosa (RP). Invest. Ophthalmol. Vis. Sci. 59, 2987 (2018).
Yang, J. et al. Translational development of human retinal progenitor cells for treatment of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 54, 2237 (2013).
Warfvinge, K. et al. Xenotransplantation of human neural progenitor cells to the subretinal space of nonimmunosuppressed pigs. J. Transplant. 2011, 948740 (2011).
Aftab, U. et al. Growth kinetics and transplantation of human retinal progenitor cells. Exp. Eye Res. 89, 301–310 (2009).
Redenti, S. et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Infor. 1, 19–29 (2008).
Singh, M. S. et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl Acad. Sci. USA 110, 1101–1106 (2013).
Garita-Hernandez, M. et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 10, 4524 (2019).
Gust, J. & Reh, T. A. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest. Ophthalmol. Vis. Sci. 52, 5266–5272 (2011).
Singh, M. S. et al. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 7, 13537 (2016).
Santos-Ferreira, T. et al. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 7, 13028 (2016).
Pearson, R. A. et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 7, 13029 (2016).
Ortin-Martinez, A. et al. A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells 35, 932–939 (2017).
Garweg, J. G., Tappeiner, C. & Halberstadt, M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv. Ophthalmol. 58, 321–329 (2013).
Li, L. & Turner, J. E. Transplantation of retinal pigment epithelial cells to immature and adult rat hosts: short- and long-term survival characteristics. Exp. Eye Res. 47, 771–785 (1988).
Royo, P. E. & Quay, W. B. Retinal transplantation from fetal to maternal mammalian eye. Growth 23, 313–336 (1959).
Kador, K. E. et al. Retinal ganglion cell polarization using immobilized guidance cues on a tissue-engineered scaffold. Acta Biomater. 10, 4939–4946 (2014).
Gandhi, J. K. et al. Fibrin hydrogels are safe, degradable scaffolds for sub-retinal implantation. PLoS ONE 15, e0227641 (2020).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Karczewski, M. & Malkiewicz, T. Scaffolds from surgically removed kidneys as a potential source of organ transplantation. Biomed. Res. Int. 2015, 325029 (2015).
Kundu, J. et al. Decellularized retinal matrix: natural platforms for human retinal progenitor cell culture. Acta Biomater. 31, 61–70 (2016).
García Delgado, A. B. et al. Subretinal transplant of induced pluripotent stem cell-derived retinal pigment epithelium on nanostructured fibrin-agarose. Tissue Eng. Part A 25, 799–808 (2019).
Krishna, Y. et al. Expanded polytetrafluoroethylene as a substrate for retinal pigment epithelial cell growth and transplantation in age-related macular degeneration. Br. J. Ophthalmol. 95, 569–573 (2011).
Chedly, J. et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 138, 91–107 (2017).
Caron, I. et al. A new three-dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 75, 135–147 (2016).
Sakiyama-Elbert, S., Johnson, P. J., Hodgetts, S. I., Plant, G. W. & Harvey, A. R. Scaffolds to promote spinal cord regeneration. Handb. Clin. Neurol. 109, 575–594 (2012).
Hong, L. T. A. et al. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 8, 533 (2017).
Masaeli, E. et al. Tissue engineering of retina through high resolution 3-dimensional inkjet bioprinting. Biofabrication 12, 025006 (2020).
Singh, D. et al. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials 154, 158–168 (2018).
Akiba, R. et al. Quantitative and qualitative evaluation of photoreceptor synapses in developing, degenerating and regenerating retinas. Front. Cell Neurosci. 13, 16 (2019).
Omori, Y. et al. Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells. J. Neurosci. 32, 6126–6137 (2012).
Nandrot, E. F., Chang, Y. & Finnemann, S. C. Alphavbeta5 integrin receptors at the apical surface of the RPE: one receptor, two functions. Adv. Exp. Med. Biol. 613, 369–375 (2008).
Dorgau, B. et al. Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids. Biomaterials 199, 63–75 (2019).
Jung, Y. H. et al. 3D microstructured scaffolds to support photoreceptor polarization and maturation. Adv. Mater. 30, e1803550 (2018).
Bernardos, R. L., Barthel, L. K., Meyers, J. R. & Raymond, P. A. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 27, 7028–7040 (2007).
Thummel, R., Kassen, S. C., Montgomery, J. E., Enright, J. M. & Hyde, D. R. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 68, 392–408 (2008).
Johns, P. R. & Fernald, R. D. Genesis of rods in teleost fish retina. Nature 293, 141–142 (1981).
Fischer, A. J. & Reh, T. A. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 4, 247–252 (2001).
Garber, K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotech. 33, 890–891 (2015).
Spencer, R., Fisher, S., Lewis, G. P. & Malone, T. Epiretinal membrane in a subject after transvitreal delivery of palucorcel (CNTO 2476). Clin. Ophthalmol. 11, 1797–1803 (2017).
Oishi, A. et al. Retinal nerve fiber layer thickness in patients with retinitis pigmentosa. Eye 23, 561–566 (2009).
Hood, D. C. et al. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 50, 2328–2336 (2009).
Punzo, C. & Cepko, C. Cellular responses to photoreceptor death in the rd1 mouse model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 48, 849–857 (2007).
MacLaren, R. E. Development and role of retinal glia in regeneration of ganglion cells following retinal injury. Br. J. Ophthalmol. 80, 458–464 (1996).
Chua, J. et al. Early remodeling of Müller cells in the rd/rd mouse model of retinal dystrophy. J. Comp. Neurol. 521, 2439–2453 (2013).
Kinouchi, R. et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 6, 863–868 (2003).
Lee, W. et al. The external limiting membrane in early-onset Stargardt disease. Invest. Ophthalmol. Vis. Sci. 55, 6139–6149 (2014).
Pearson, R. A. et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 19, 487–503 (2010).
Lao, L. L., Peppas, N. A., Boey, F. Y. & Venkatraman, S. S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 418, 28–41 (2011).
Singh, R. K., Kolandaivelu, S. & Ramamurthy, V. Early alteration of retinal neurons in Aipl1−/− animals. Invest. Ophthalmol. Vis. Sci. 55, 3081–3092 (2014).
Tansley, K. The development of the rat eye in graft. J. Exp. Biol. 22, 221–223 (1946).
Humayun, M. S. et al. Human neural retinal transplantation. Invest. Ophthalmol. Vis. Sci. 41, 3100–3106 (2000).
Tomita, M. et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 20, 279–283 (2002).
MacLaren, R. E. et al. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207 (2006).
Lamba, D. A., Gust, J. & Reh, T. A. Transplantation of human embryonic stem cell derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79 (2009).
Lamba, D. A. et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 5, e8763 (2010).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Pearson, R. A. et al. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103 (2012).
Howden, S. E. et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc. Natl Acad. Sci. USA 108, 6537–6542 (2011).
Barnea-Cramer, A. O. et al. Repair of retinal degeneration following ex vivo minicircle DNA gene therapy and transplantation of corrected photoreceptor progenitors. Mol. Ther. 28, 830–844 (2020).
Naldini, L. Ex vivo gene transfer and correction for cell-based therapies. Nat. Rev. Genet. 12, 301–315 (2011).
Chow, A. Y. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch. Ophthalmol. 122, 460–469 (2004).
Guenther, T., Lovell, N. H. & Suaning, G. J. Bionic vision: system architectures: a review. Expert Rev. Med. Devices 9, 33–48 (2012).
Garg, S. J. & Federman, J. Optogenetics, visual prosthesis and electrostimulation for retinal dystrophies. Curr. Opin. Ophthalmol. 24, 407–414 (2013).
Luo, Y. H. & da Cruz, L. A review and update on the current status of retinal prostheses (bionic eye). Br. Med. Bull. 109, 31–44 (2014).
Zrenner, E. Fighting blindness with microelectronics. Sci. Transl. Med. 5, 210ps16 (2013).
Humayun, M. S. et al. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vis. Res. 43, 2573–2581 (2003).
Yanai, D. et al. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am. J. Ophthalmol. 143, 820–827 (2007).
Rizzo, J. F. III, Wyatt, J., Loewenstein, J., Kelly, S. & Shire, D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest. Ophthalmol. Vis. Sci. 44, 5362–5369 (2003).
Humayun, M. S. et al. Preliminary 6 month results from the Argus II epiretinal prosthesis feasibility study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 4566–4568 (2009).
Ho, A. C. et al. Argus II Study Group. LONG-term results from an epiretinal prosthesis to restore sight to the blind. Ophthalmology 122, 1547–1554 (2015).
Humayun, M. S. et al. Argus II Study Group. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 119, 779–788 (2012).
Richard, G. et al. Multicenter study on acute electrical stimulation of the human retina with an epiretinal implant: clinical results in 20 patients. Invest. Ophthalmol. Vis. Sci. 46, 1143 (2005).
Keserü, M. et al. Acute electrical stimulation of the human retina with an epiretinal electrode array. Acta Ophthalmol. 90, e1–e8 (2012).
da Cruz, L. et al. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Argus II Study Group. Ophthalmology 123, 2248–2254 (2016).
Zrenner, E. et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc. Biol. Sci. 278, 1489–1497 (2011).
Roessler, G. et al. Implantation and explantation of a wireless epiretinal retina implant device in blind RP patients. Invest. Ophthalmol. Vis. Sci. 50, 3003–3008 (2009).
Stingl, K. et al. Subretinal visual implant Alpha IMS–clinical trial interim report. Vis. Res. 111, 149–160 (2015).
Stingl, K. et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. Biol. Sci. 280, 20130077 (2013).
Stingl, K. et al. Interim results of a multicenter trial with the new electronic subretinal implant Alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci. 11, 445 (2017).
Edwards, T. L. et al. Assessment of the electronic retinal implant Alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology 125, 432–443 (2018).
Ayton, L. N. et al. Bionic Vision Australia Research Consortium. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE 9, e115239 (2014).
Fujikado, T. et al. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 52, 4726–4733 (2011).
Abbott, C. J. et al. Safety studies for a 44-channel suprachoroidal retinal prosthesis: a chronic passive study. Invest. Ophthalmol. Vis. Sci. 59, 1410–1424 (2018).
MacLaren, R. E. Electronic retinal implant surgery. Eye 31, 191–195 (2017).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 6, 391–397 (2012).
Perez Fornos, A., Sommerhalder, J., Pittard, A., Safran, A. B. & Pelizzone, M. Simulation of artificial vision: IV. Visual information required to achieve simple pointing and manipulation tasks. Vis. Res. 48, 1705–1718 (2008).
Palanker, D., Le Mer, Y., Mohand-Said, S., Muqit, M. & Sahel, J. A. Photovoltaic restoration of central vision in atrophic age-related macular degeneration. Ophthalmology 127, 1097–1104 (2020).
Duncan, J. L. et al. Improvements in vision-related quality of life in blind patients implanted with the Argus II epiretinal prosthesis. Clin. Exp. Optom. 100, 144–150 (2017).
Dagnelie, G. et al. Argus® II Study Group. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus® II retinal prosthesis system. Clin. Exp. Ophthalmol. 45, 152–159 (2017).
da Cruz, L. et al. Argus II Study Group. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254 (2016).
Luo, Y. H., Zhong, J. J. & da Cruz, L. The use of Argus® II retinal prosthesis by blind subjects to achieve localisation and prehension of objects in 3-dimensional space. Graefes Arch. Clin. Exp. Ophthalmol. 253, 1907–1914 (2015).
Kotecha, A., Zhong, J., Stewart, D. & da Cruz, L. The Argus II prosthesis facilitates reaching and grasping tasks: a case series. BMC Ophthalmol. 14, 71 (2014).
da Cruz, L. et al. Argus II Study Group. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br. J. Ophthalmol. 97, 632–636 (2013).
Dorn, J. D. et al. Argus II Study Group. The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol. 131, 183–189 (2013).
Geruschat, D. R. et al. An analysis of observer-rated functional vision in patients implanted with the Argus II retinal prosthesis system at three years. Clin. Exp. Optom. 99, 227–232 (2016).
Jensen, R. J., Rizzo, J. F. III, Ziv, O. R., Grumet, A. & Wyatt, J. Thresholds for activation of rabbit retinal ganglion cells with an ultrafine, extracellular microelectrode. Invest. Ophthalmol. Vis. Sci. 44, 3533–3543 (2003).
Brindley, G. S. & Lewin, W. S. The visual sensations produced by electrical stimulation of the medial occipital cortex. J. Physiol. 194, 54-5P (1968).
Dobelle, W. H., Mladejovsky, M. G. & Girvin, J. P. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 183, 440–444 (1974).
Lewis, P. M. & Rosenfeld, J. V. Electrical stimulation of the brain and the development of cortical visual prostheses: an historical perspective. Brain Res. 1630, 208–224 (2016).
Bradley, D. C. et al. Visuotopic mapping through a multichannel stimulating implant in primate V1. J. Neurophys. 93, 1659–1670 (2005).
Niketeghad, S. & Pouratian, N. Brain machine interfaces for vision restoration: the current state of cortical visual prosthetics. Neurotherapeutics 16, 134–143 (2019).
Rosenfeld, J. V. et al. Tissue response to a chronically implantable wireless intracortical visual prosthesis (Gennaris array). J. Neural Eng. 17, 046001 (2020).
Fernández, E & Normann, R. in Artificial Vision (ed. Gabel, V. P.) 191–201 (Springer, 2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Zhu, J. et al. Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors. Cell Res. 27, 830–833 (2017).
Edwards, T. L. et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat. Biomed. Eng. 2, 649–656 (2018).
Cehajic-Kapetanovic, J. et al. First-in-human robot-assisted subretinal drug delivery under local anaesthesia: a randomisedclinical trial. Am. J. Ophthalmol. https://doi.org/10.1016/j.ajo.2021.11.011 (2021).
Mayoral, S. R., Etxeberria, A., Shen, Y. A. & Chan, J. R. Initiation of CNS myelination in the optic nerve is dependent on axon caliber. Cell Rep. 25, 544–550.e3 (2018).
Ayton, L. N. et al. Harmonization of outcomes and vision endpoints in vision restoration trials: recommendations from the International HOVER Taskforce. Transl. Vis. Sci. Technol. 9, 25 (2020).
Maya-Vetencourt, J. F. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 16, 681–689 (2017).
Ha, S. et al. Towards high-resolution retinal prostheses with direct optical addressing and inductive telemetry. J. Neural Eng. 13, 056008 (2016).
Samba, R., Herrmann, T. & Zeck, G. PEDOT-CNT coated electrodes stimulate retinal neurons at low voltage amplitudes and low charge densities. J. Neural Eng. 12, 016014 (2015).
Jones, P. D. & Stelzle, M. Can nanofluidic chemical release enable fast, high resolution neurotransmitter-based neurostimulation? Front. Neurosci. 10, 138 (2016).
Albert, E. S. et al. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J. Neurophysiol. 107, 3227–3234 (2012).
Bonmassar, G. et al. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 3, 921 (2012).
Holladay, J. T. Visual acuity measurements. J. Cataract Refract. Surg. 30, 287–290 (2004).
Acknowledgements
We thank J. Brett (BA Arts and Graphics) and T. Buckley (BM BCh) from Eye Research Group Oxford for their help with the images and figure design. We acknowledge funding from the University of Oxford NIHR Biomedical Research Centre. J.C.-K. was also funded by the Global Ophthalmology Fellowship Awards Programme, Bayer, Switzerland. E.Z. was supported by the DFG excellence center EXC 307 (CIN Tübingen). R.E.M. was funded by an MRC i4i Award for clinical trials of retinal implants.
Author information
Authors and Affiliations
Contributions
J.C.-K. wrote and revised the manuscript, with input from E.Z. on section ‘Retinal and cortical prostheses’, M.S.S. on section ‘Cell therapy’, and R.E.M. on the overall manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Budd Tucker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cehajic-Kapetanovic, J., Singh, M.S., Zrenner, E. et al. Bioengineering strategies for restoring vision. Nat. Biomed. Eng 7, 387–404 (2023). https://doi.org/10.1038/s41551-021-00836-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00836-4
This article is cited by
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)