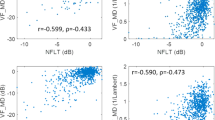
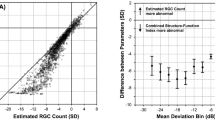
Abstract
The clinical diagnostic evaluation of optic neuropathies relies on the analysis of the thickness of the retinal nerve fibre layer (RNFL) by optical coherence tomography (OCT). However, false positives and false negatives in the detection of RNFL abnormalities are common. Here we show that an algorithm integrating measurements of RNFL thickness and reflectance from standard wide-field OCT scans can be used to uncover the trajectories and optical texture of individual axonal fibre bundles in the retina and to discern distinctive patterns of loss of axonal fibre bundles in glaucoma, compressive optic neuropathy, optic neuritis and non-arteritic anterior ischaemic optic neuropathy. Such optical texture analysis can detect focal RNFL defects in early optic neuropathy, as well as residual axonal fibre bundles in end-stage optic neuropathy that were indiscernible by conventional OCT analysis and by red-free RNFL photography. In a diagnostic-performance study, optical texture analysis of the RNFL outperformed conventional OCT in the detection of glaucoma, as defined by visual-field testing or red-free photography. Our findings show that optical texture analysis of the RNFL for the detection of optic neuropathies is highly sensitive and specific.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All patient data are available from the corresponding author on reasonable request, subject to approval from the Institutional Review Board of Hong Kong Hospital Authority research ethics committees. The Cirrus OCT normative data have been described22.
Code availability
Conventional texture-based image analyses were performed using MATLAB R2018a. The texture features required for GLCM texture analysis were extracted using functions from Statistics and Machine Learning Toolbox (MATLAB R2018a), Image Processing Toolbox (MATLAB R2018a) and code (GLCMFeatures ver. 2.1.1.0 by Patrik Brynolfsso 2016, publicly available from https://www.mathworks.com/matlabcentral/fileexchange/55034-glcmfeatures-glcm). The texture feature required for wavelet texture analysis was extracted using functions from Image Processing Toolbox (MATLAB R2018a). ROTA images were generated using a custom code developed in MATLAB R2018a. The code is available from the corresponding author on reasonable request. Statistical analysis was performed with STATA (ver. 15) and R (ver. 3.4.4).
References
Sommer, A. et al. Evaluation of nerve fiber layer assessment. Arch. Ophthalmol. 102, 1766–1771 (1984).
O’Neill, E. C. et al. The optic nerve head in acquired optic neuropathies. Nat. Rev. Neurol. 6, 221–236 (2010).
Weinreb, R. N. et al. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2, 16067 (2016).
Hood, D. C. Improving our understanding, and detection, of glaucomatous damage: an approach based upon optical coherence tomography (OCT). Prog. Retin. Eye Res. 57, 46–75 (2017).
Micieli, J. A., Newman, N. J. & Biousse, V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr. Opin. Neurol. 32, 115–123 (2019).
Mutlu, U. et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 75, 1256–1263 (2018).
Petzold, A. et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 16, 797–812 (2017).
Ahn, J. et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 91, e1003–e1012 (2018).
Andrade, C. et al. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s disease. Mov. Disord. 31, 377–383 (2016).
Doustar, J., Torbati, T., Black, K. L., Koronyo, Y. & Koronyo-Hamaoui, M. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front. Neurol. 8, 701 (2017).
Leung, C. K. et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology 116, 1257–1263 (2009).
Mwanza, J. C. et al. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest. Ophthalmol. Vis. Sci. 52, 8323–8329 (2011).
Oddone, F. et al. Macular versus retinal nerve fiber layer parameters for diagnosing manifest glaucoma: a systematic review of diagnostic accuracy studies. Ophthalmology 123, 939–949 (2016).
Leung, C. K. & Lam, A. K. Optical texture analysis of the inner retina. US patent 62/571,559 (2017).
Flaxman, S. R. et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob. Health 5, e1221–e1234 (2017).
Cohen, J. F. et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6, e012799 (2016).
Pons, M. E. et al. Assessment of retinal nerve fiber layer internal reflectivity in eyes with and without glaucoma using optical coherence tomography. Arch. Ophthalmol. 118, 1044–1047 (2000).
Vermeer, K. A., van der Schoot, J., Lemij, H. G. & de Boer, J. F. RPE-normalized RNFL attenuation coefficient maps derived from volumetric OCT imaging for glaucoma assessment. Invest. Ophthalmol. Vis. Sci. 53, 6102–6108 (2012).
Greenfield, D. S. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin. Ophthalmol. 14, 95–108 (1999).
Mcleod, D. Pathogenesis of optic disc swelling. Br. J. Ophthalmol. 62, 579–580 (1978).
Biswas, S., Lin, C. & Leung, C. K. Evaluation of a myopic normative database for analysis of retinal nerve fiber layer thickness. JAMA Ophthalmol. 134, 1032–1039 (2016).
Knight, O. J. et al. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch. Ophthalmol. 130, 312–318 (2012).
Hood, D. C. et al. Details of glaucomatous damage are better seen on OCT en face images than on OCT retinal nerve fiber layer thickness maps. Invest. Ophthalmol. Vis. Sci. 56, 6208–6216 (2015).
Chauhan, B. C., Sharpe, G. P. & Hutchison, D. M. Imaging of the temporal raphe with optical coherence tomography. Ophthalmology 121, 2287–2288 (2014).
Dong, Z. M., Wollstein, G., Wang, B. & Schuman, J. S. Adaptive optics optical coherence tomography in glaucoma. Prog. Retin. Eye Res. 57, 76–88 (2017).
Hood, D. C. et al. Confocal adaptive optics imaging of peripapillary nerve fiber bundles: implications for glaucomatous damage seen on circumpapillary OCT scans. Transl. Vis. Sci. Technol. 4, 12 (2015).
Bae, H. W. et al. Comparison of three types of images for the detection of retinal nerve fiber layer defects. Optom. Vis. Sci. 92, 500–505 (2015).
Neelam, K., Cheung, C. M., Ohno-Matsui, K., Lai, T. Y. & Wong, T. Y. Choroidal neovascularization in pathological myopia. Prog. Retin. Eye Res. 31, 495–525 (2012).
CCRB Clinical Trials Registry, CUHK_CCRB00439. Progressive Lamina Cribrosa Deformation – A Biomarker for Fast Progressors in Glaucoma (The Chinese University of Hong Kong, 2014); https://www2.ccrb.cuhk.edu.hk/registry/public/278
CCRB Clinical Trials Registry, CUHK_CCRB00591. Measurement of the Rates of Retinal Nerve Fiber Layer Thinning to Guide Management of Glaucoma Patients (The Chinese University of Hong Kong, 2014); https://www2.ccrb.cuhk.edu.hk/registry/public/457
ANZCTR, ACTRN12618000453280. Progressive Retinal Nerve Fiber Layer (RNFL) Thinning as a Biomarker to Guide Intraocular Pressure (IOP) Lowering Treatment in Ocular Hypertensives (OHT). (ANZCTR, 2018); https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373418
Leung, C.K. in Diagnosis of Primary Open Angle Glaucoma (eds Weinreb, R. N., Leung, C. K., Garway-Heath, D. F., Medeiros, F. A. & Liebmann, J.) 1–20 (WGA Consensus Series 10, Kugler, 2016).
Leung, C. K. et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: analysis of the retinal nerve fiber layer map for glaucoma detection. Ophthalmology 117, 1684–1691 (2010).
Haralick, R. M., Shanmugam, K. & Dinstein, I. Textural features for image classification. IEEE Trans. Syst. Man Cybern. SMC-3, 610–621 (1973).
Bovik, A. C., Clark, M. & Geisler, W. S. Multichannel texture analysis using localized spatial filters. IEEE Trans. Pattern Anal. Mach. Intell. 12, 55–73 (1990).
Anitha, J. & Peter, J. D. A wavelet based morphological mass detection and classification in mammograms. In International Conference on Machine Vision and Image Processing (MVIP) 25–28 (2012).
Ben Salem, Y. & Nasri, S. Automatic recognition of woven fabrics based on texture and using SVM. Signal Image Video Process. 4, 429–434 (2010).
Kandaswamy, U., Adjeroh, D. A. & Lee, M. C. Efficient texture analysis of SAR imagery. IEEE Trans. Geosci. Remote Sens. 43, 2075–2083 (2005).
Bharati, M. H., Liu, J. J. & MacGregor, J. F. Image texture analysis: methods and comparisons. Chemometr. Intell. Lab. Syst. 72, 57–71 (2004).
Youden, W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950).
Yang, Z., Sun, X. & Hardin, J. W. A note on the tests for clustered matched-pair binary data. Biom. J. 52, 638–652 (2010).
Obuchowski, N. A. On the comparison of correlated proportions for clustered data. Stat. Med. 17, 1495–1507 (1998).
Pepe, M. S. Three approaches to regression analysis of receiver operating characteristic curves for continuous test results. Biometrics 54, 124–135 (1998).
Acknowledgements
This work was supported by the Hong Kong Research Grants Council General Research Fund 14101518, 14101117, 14100916 and 14101215.
Author information
Authors and Affiliations
Contributions
C.K.S.L. conceptualized and designed the study; C.K.S.L. and A.K.N.L. developed the algorithm of ROTA; K.H.N.W., M.W., C.Y.L.C., C.K.M.C., N.C.Y.C., K.W.K. and C.K.S.L. recruited study participants; C.K.S.L., M.Y., A.K.N.L. and P.Y.G. performed data analysis; C.K.S.L. wrote the manuscript; all authors discussed the results, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.K.S.L. and A.K.N.L. hold a patent (US patent 62/571,559) for ROTA and are founders of AIROTA Diagnostics Limited. A licensing agreement is under discussion between the Chinese University of Hong Kong and Heidelberg Engineering (Heidelberg, Germany) and Carl Zeiss Meditec (Dublin, CA, United States). C.K.S.L. has received research support in the form of instruments, research grants and speaker honoraria from Carl Zeiss Meditec, Heidelberg Engineering and Topcon (Tokyo, Japan). R.N.W. has received instruments from Carl Zeiss Meditec, Heidelberg Engineering and Optovue (Fremont, CA, United States).
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, results, figures and tables.
Rights and permissions
About this article
Cite this article
Leung, C.K.S., Lam, A.K.N., Weinreb, R.N. et al. Diagnostic assessment of glaucoma and non-glaucomatous optic neuropathies via optical texture analysis of the retinal nerve fibre layer. Nat. Biomed. Eng 6, 593–604 (2022). https://doi.org/10.1038/s41551-021-00813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00813-x
This article is cited by
-
Comparison of the retinal microvasculature between compressive and glaucomatous optic neuropathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)