Abstract
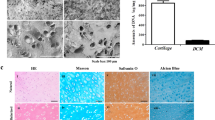
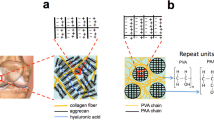
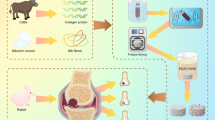
The early stages of progressive degeneration of cartilage in articular joints are a hallmark of osteoarthritis. Healthy cartilage is lubricated by brush-like cartilage-binding nanofibres with a hyaluronan backbone and two key side chains (lubricin and lipid). Here, we show that hyaluronan backbones grafted with lubricin-like sulfonate-rich polymers or with lipid-like phosphocholine-rich polymers together enhance cartilage regeneration in a rat model of early osteoarthritis. These biomimetic brush-like nanofibres show a high affinity for cartilage proteins, form a lubrication layer on the cartilage surface and efficiently lubricate damaged human cartilage, lowering its friction coefficient to the low levels typical of native cartilage. Intra-articular injection of the two types of nanofibre into rats with surgically induced osteoarthritic joints led to cartilage regeneration and to the abrogation of osteoarthritis within 8 weeks. Biocompatible injectable lubricants that facilitate cartilage regeneration may offer a translational strategy for the treatment of early osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its supplementary information. The raw and analysed datasets generated during the study are too large to be publicly shared; however, they are available for research purposes from the corresponding authors upon reasonable request.
References
Wieland, H. A., Michaelis, M., Kirschbaum, B. J. & Rudolphi, K. A. Osteoarthritis—an untreatable disease? Nat. Rev. Drug Discov. 4, 331–344 (2005).
Li, M. H., Xiao, R., Li, J. B. & Zhu, Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthritis Cartilage 25, 1577–1587 (2017).
He, Z., Wang, B., Hu, C. & Zhao, J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloid Surf. B 154, 33–39 (2017).
Morgese, G., Benetti, E. M. & Zenobi-Wong, M. Molecularly engineered biolubricants for articular cartilage. Adv. Healthc. Mater. 7, 1701463 (2018).
Goldring, S. R. & Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 12, 632–644 (2016).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Morgese, G., Cavalli, E., Muller, M., Zenobi-Wong, M. & Benetti, E. M. Nanoassemblies of tissue-reactive, polyoxazoline graft-copolymers restore the lubrication properties of degraded cartilage. ACS Nano 11, 2794–2804 (2017).
Samaroo, K. J., Tan, M., Putnam, D. & Bonassar, L. J. Binding and lubrication of biomimetic boundary lubricants on articular cartilage. J. Orthop. Res. 35, 548–557 (2017).
Morgese, G., Cavalli, E., Rosenboom, J. G., Zenobi-Wong, M. & Benetti, E. M. Cyclic polymer grafts that lubricate and protect damaged cartilage. Angew. Chem. Int. Ed. 57, 1621–1626 (2018).
Singh, A. et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 13, 988–995 (2014).
Lawrence, A. et al. Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface. Biomaterials 73, 42–50 (2015).
Prudnikova, K. et al. Biomimetic proteoglycans mimic macromolecular architecture and water uptake of natural proteoglycans. Biomacromolecules 18, 1713–1723 (2017).
Banquy, X., Burdynska, J., Lee, D. W., Matyjaszewski, K. & Israelachvili, J. Bioinspired bottle-brush polymer exhibits low friction and Amontons-like behavior. J. Am. Chem. Soc. 136, 6199–6202 (2014).
Faivre, J. et al. Intermolecular interactions between bottlebrush polymers boost the protection of surfaces against frictional. Wear. Chem. Mat. 30, 4140–4149 (2018).
Klein, J. Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 220, 691–710 (2006).
Banquy, X., Lee, D. W., Das, S., Hogan, J. & Israelachvili, J. N. Shear-induced aggregation of mammalian synovial fluid components under boundary lubrication conditions. Adv. Funct. Mater. 24, 3152–3161 (2014).
Seror, J. et al. Normal and shear interactions between hyaluronan–aggrecan complexes mimicking possible boundary lubricants in articular cartilage in synovial joints. Biomacromolecules 13, 3823–3832 (2012).
Seror, J. et al. Articular cartilage proteoglycans as boundary lubricants: structure and frictional interaction of surface-attached hyaluronan and hyaluronan–aggrecan complexes. Biomacromolecules 12, 3432–3443 (2011).
Maeda, S., Hara, Y., Sakai, T., Yoshida, R. & Hashimoto, S. Self-walking gel. Adv. Mater. 19, 3480–3484 (2007).
Means, A. K., Shrode, C. S., Whitney, L. V., Ehrhardt, D. A. & Grunlan, M. A. Double network hydrogels that mimic the modulus, strength, and lubricity of cartilage. Biomacromolecules 20, 2034–2042 (2019).
Ishihara, K. Highly lubricated polymer interfaces for advanced artificial hip joints through biomimetic design. Polym. J. 47, 585–597 (2015).
Laterra, J., Silbert, J. E. & Culp, L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J. Cell Biol. 96, 112–123 (1983).
Rossi, J. D., & Wallace, B. A. Binding of fibronectin to phospholipid vesicles. J. Biol. Chem. 258, 3327–3331 (1983).
Heremans, A., de Cock, B, Cassiman, J. J., Van den Berghe, H. & David, G. The core protein of the matrix-associated heparan sulfate proteoglycan binds to fibronectin. J. Biol. Chem. 285, 8716–8724 (1990).
Oh, E. J. et al. Control of the molecular degradation of hyaluronic acid hydrogels for tissue augmentation. J. Biomed. Mater. Res. Part A 86, 685–693 (2008).
Jahn, S., Seror, J. & Klein, J. Lubrication of articular cartilage. Annu. Rev. Biomed. Eng. 18, 235–258 (2016).
Klein, J. Hydration lubrication. Friction 1, 1–23 (2013).
Silbert, G., Kampf, N. & Klein, J. Normal and shear forces between charged solid surfaces immersed in cationic surfactant solution: the role of the alkyl chain length. Langmuir 30, 5097–5104 (2014).
Su, K., Lau, T. T., Leong, W., Gong, Y. & Wang, D.-A. Creating a living hyaline cartilage graft free from non-cartilaginous constituents: an intermediate role of a biomaterial scaffold. Adv. Funct. Mater. 22, 972–978 (2012).
Lee, H. P., Gu, L., Mooney, D. J., Levenston, M. E. & Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243–1251 (2017).
Lorenz, H., Wenz, W., Ivancic, M., Steck, E. & Richter, W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res. Ther. 7, 156–165 (2005).
Inada, M. et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl Acad. Sci. USA 101, 17192–17197 (2004).
Desando, G. et al. Short-term homing of hyaluronan-primed cells: therapeutic implications for osteoarthritis treatment. Tissue Eng. Part C 24, 121–133 (2018).
Ishikawa, M. et al. Biocompatibility of cross-linked hyaluronate (Gel-200) for the treatment of knee osteoarthritis. Osteoarthr. Cartil. 22, 1902–1909 (2014).
Yoshioka, K. et al. Biocompatibility study of different hyaluronan products for intra-articular treatment of knee osteoarthritis. BMC Musculoskel. Dis. 20, 424 (2019).
Vincent, T. L. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr. Opin. Pharmacol. 13, 449–454 (2013).
Meinert, C. et al. Tailoring hydrogel surface properties to modulate cellular response to shear loading. Acta Biomater. 52, 105–117 (2017).
Bonnevie, E. D. et al. Microscale frictional strains determine chondrocyte fate in loaded cartilage. J. Biomech. 74, 72–78 (2018).
Jin, M., Frank, E. H., Quinn, T. M., Hunziker, E. B. & Grodzinsky, A. J. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch. Biochem. Biophys. 395, 41–48 (2001).
Kellum, M. G., Harris, C. A., Mccormick, C. L. & Morgan, S. E. Stimuli-responsive micelles of amphiphilic AMPS-b-AAL copolymers in layer-by-layer films. J. Polym. Sci. Pol. Chem. 49, 1104–1111 (2011).
Kellum, M. G., Smith, A. E., York, S. K. & McCormick, C. L. Reversible interpolyelectrolyte shell cross-linked micelles from pH/salt-responsive diblock copolymers synthesized via RAFT in aqueous solution. Macromolecules 43, 7033–7040 (2010).
Bhuchar, N., Deng, Z., Ishihara, K. & Narain, R. Detailed study of the reversible addition–fragmentation chain transfer polymerization and co-polymerization of 2-methacryloyloxyethyl phosphorylcholine. Polym. Chem. 2, 632–639 (2011).
Chan, J. W., Yu, B., Hoyle, C. E. & Lowe, A. B. Convergent synthesis of 3-arm star polymers from RAFT-prepared poly(N,N-diethylacrylamide) via a thiol-ene click reaction. Chem. Commun. 40, 4959–4961 (2008).
Korogiannaki, M., Zhang, J. & Sheardown, H. Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics. J. Biomater. Appl. 32, 446–462 (2017).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Case, D. A. et al. The amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Wang, J., Wang, W., Kollman, P. A. & Case, D. A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. 25, 247–260 (2006).
Ryckaert, J.P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the cartesian equations of motion of a system with constraints molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Miller, B. R. III et al. MMPBSA.py: an efficient program for end-state free energy calculations. J. Chem. Theory Comput. 8, 3314–3321 (2012).
Schmidt, T. A. & Sah, R. L. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthr. Cartil. 15, 35–47 (2007).
Ko, J. Y., Choi, Y. J., Jeong, G. J. & Im, G. I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 34, 5359–5368 (2013).
Kang, M. L., Ko, J. Y., Kim, J. E. & Im, G. I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35, 9984–9994 (2014).
Feng, Q. et al. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 53, 329–342 (2017).
Acknowledgements
We sincerely thank X. Miao from South China University of Technology for her contributions to the AFM characterizations. We also sincerely appreciate the help and guidance of F. Zhang and S. Jiang from Sun Yat-Sen University for the anterior-cruciate-ligament-transection-induced OA surgery. S.L. and L.R. are thankful for financial support from the National Natural Science Foundation of China (51673071), the Natural Science Foundation of Guangdong Province (2016A030313509), the Guangdong Scientific and Technological Project (2014B090907004), and the National Key Research and Development Program of China (2017YFC1105004). Y.Z. and C.M. would like to acknowledge support from the Institute for Biomedical Engineering, Science and Technology of the University of Oklahoma.
Author information
Authors and Affiliations
Contributions
R.X., S.L., L.R. and C.M. supervised the project. H.Y. carried out the synovial stem cell experiments under the supervision of D.-A.W. R.X., H.Y., A.S.M., Y.Z., Y.J., M.G., Y.C. and L.W. carried out the rest of the experiments and characterization. D.Q. assisted in analysing the confocal results, and K.W. provided fruitful discussion in the results of the animal experiments. A.S.M. designed the illustrations and edited the writing. All the authors contributed to the discussions and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and figures.
Rights and permissions
About this article
Cite this article
Xie, R., Yao, H., Mao, A.S. et al. Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nat Biomed Eng 5, 1189–1201 (2021). https://doi.org/10.1038/s41551-021-00785-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00785-y
This article is cited by
-
Utilizing bioprinting to engineer spatially organized tissues from the bottom-up
Stem Cell Research & Therapy (2024)
-
Bioinspired surface functionalization of biodegradable mesoporous silica nanoparticles for enhanced lubrication and drug release
Friction (2023)
-
Tribology of enzymatically degraded cartilage mimicking early osteoarthritis
Friction (2023)
-
Fluorinated graphene quantum dots with long-term lubrication for visual drug loading and joint inflammation therapy
Friction (2023)
-
Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis
Nature Reviews Rheumatology (2022)