Abstract
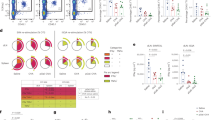
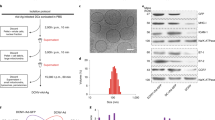
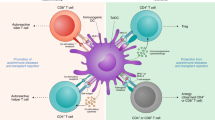
The association of autoimmune diseases with particular allellic products of the class-II major histocompatibility complex (MHCII) region implicates the presentation of the offending self-antigens to T cells. Because antigen-presenting cells are tolerogenic when they encounter an antigen under non-inflammatory conditions, the manipulation of antigen presentation may induce antigen-specific tolerance. Here, we show that, in mouse models of experimental autoimmune encephalomyelitis, type 1 diabetes and rheumatoid arthritis, the systemic administration of a single dose of nanobodies that recognize MHCII molecules and conjugated to the relevant self-antigen under non-inflammatory conditions confers long-lasting protection against these diseases. Moreover, co-administration of a nanobody–antigen adduct and the glucocorticoid dexamethasone, conjugated to the nanobody via a cleavable linker, halted the progression of established experimental autoimmune encephalomyelitis in symptomatic mice and alleviated their symptoms. This approach may represent a means of treating autoimmune conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results of this study, including the nanobody sequences used, are available within the paper and its Supplementary Information. The transcriptomic datasets generated during the current study are available from the corresponding authors upon reasonable request.
References
Hayter, S. M. & Cook, M. C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 11, 754–765 (2012).
Morel, L. Mouse models of human autoimmune diseases: essential tools that require the proper controls. PLoS Biol. 2, E241 (2004).
Bonifaz, L. C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Idoyaga, J. et al. Comparable T helper 1 (TH1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc. Natl Acad. Sci. USA 108, 2384–2389 (2011).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Duarte, J. N. et al. Generation of immunity against pathogens via single-domain antibody–antigen constructs. J. Immunol. 197, 4838–4847 (2016).
Van Elssen, C. et al. Noninvasive imaging of human immune responses in a human xenograft model of graft-versus-host disease. J. Nucl. Med. 58, 1003–1008 (2017).
Fang, T. et al. Structurally defined αMHC-II nanobody–drug conjugates: a therapeutic and imaging system for B-cell lymphoma. Angew. Chem. Int. Ed. Engl. 55, 2416–2420 (2016).
Ingram, J. R., Schmidt, F. I. & Ploegh, H. L. Exploiting nanobodies’ singular traits. Annu. Rev. Immunol. 36, 695–715 (2018).
Pishesha, N., Ingram, J. R. & Ploegh, H. L. Sortase A: a model for transpeptidation and its biological applications. Annu. Rev. Cell Dev. Biol. 34, 163–188 (2018).
Villadangos, J. A. & Schnorrer, P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7, 543–555 (2007).
Giralt, M., Molinero, A. & Hidalgo, J. Active induction of experimental autoimmune encephalomyelitis (EAE) with MOG35-55 in the mouse. Methods Mol. Biol. 1791, 227–232 (2018).
Boes, M. et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418, 983–988 (2002).
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Isaksson, M. et al. Conditional DC depletion does not affect priming of encephalitogenic TH cells in EAE. Eur. J. Immunol. 42, 2555–2563 (2012).
Bodhankar, S. et al. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur. J. Immunol. 41, 1165–1175 (2011).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Ruffo, E. et al. Lymphocyte-activation gene 3 (LAG3): the next immune checkpoint receptor. Semin. Immunol. 42, 101305 (2019).
Thaker, Y. R. et al. Treg-specific LAG3 deletion reveals a key role for LAG3 in regulatory T cells to inhibit CNS autoimmunity. J. Immunol. 200, 101.7 (2018).
Paterson, A. M. et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 212, 1603–1621 (2015).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Katz, J. D. et al. Following a diabetogenic T cell from genesis through pathogenesis. Cell 74, 1089–1100 (1993).
Maffia, P. et al. Inducing experimental arthritis and breaking self-tolerance to joint-specific antigens with trackable, ovalbumin-specific T cells. J. Immunol. 173, 151–156 (2004).
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Sehrawat, S. et al. CD8+ T cells from mice transnuclear for a TCR that recognizes a single H-2Kb-restricted MHV68 epitope derived from gB-ORF8 help control infection. Cell Rep. 1, 461–471 (2012).
Avalos, A. M. et al. Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J. Exp. Med. 211, 365–379 (2014).
Bargh, J. D. et al. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Florez-Grau, G. et al. Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front Immunol. 9, 1169 (2018).
Sharrack, B. et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transpl. 55, 283–306 (2020).
Pishesha, N. et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc. Natl Acad. Sci. USA 114, 3157–3162 (2017).
Wilson, D. S. et al. Synthetically glycosylated antigens induce antigen-specific tolerance and prevent the onset of diabetes. Nat. Biomed. Eng. 3, 817–829 (2019).
Kontos, S. et al. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl Acad. Sci. USA 110, E60–E68 (2013).
Lorentz, K. M. et al. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci. Adv. 1, e1500112 (2015).
Kishimoto, T. K. & Maldonado, R. A. Nanoparticles for the induction of antigen-specific immunological tolerance. Front. Immunol. 9, 230 (2018).
Pearson, R. M. et al. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Adv. Drug Deliv. Rev. 114, 240–255 (2017).
Iberg, C. A., Jones, A. & Hawiger, D. Dendritic cells as inducers of peripheral tolerance. Trends Immunol. 38, 793–804 (2017).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Henderson, J. G., Opejin, A., Jones, A., Gross, C. & Hawiger, D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity 42, 471–483 (2015).
Vanderlugt, C. J. & Miller, S. D. Epitope spreading. Curr. Opin. Immunol. 8, 831–836 (1996).
Unanue, E. R. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu. Rev. Immunol. 32, 579–608 (2014).
McCombs, J. R. & Owen, S. C. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 17, 339–351 (2015).
Nicolaou, K. C. & Rigol, S. The role of organic synthesis in the emergence and development of antibody–drug conjugates as targeted cancer therapies. Angew. Chem. Int. Ed. Engl. 58, 11206–11241 (2019).
Rashidian, M. et al. Noninvasive imaging of immune responses. Proc. Natl Acad. Sci. USA 112, 6146–6151 (2015).
Acknowledgements
This work is supported by a post-doctoral Junior Fellowship from the Harvard Society of Fellows (to N.P.), a mobility fellowship from the Swiss National Science Foundation (grant number P400P2_183857 to T.H.), a scholarship from the Dutch MS Research Foundation (to L.Y.S. and R.J.), an overseas grant from UiT The Arctic University of Norway (to A.I.) and an NIH Director’s Pioneer Award (grant number 1DP1AI150593-01 to H.L.P.). We also thank A. Regev for helpful discussions and laboratory equipment access.
Author information
Authors and Affiliations
Contributions
N.P. and H.L.P. designed and conceived of the study. N.P., T.H., L.Y.S., W.M. and R.J. contributed to data acquisition and analysed and interpreted the data. L.S.L., A.I., Y.J.X., T.F., N.M., W.P. III, H.R.S., M.A.R. and G.G.-S. contributed to data acquisition. N.P., L.Y.S., R.J. and H.L.P. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The Boston Children’s Hospital has filed for patent protection (US Provisional Application Serial No. 63/154,455) on the technology described here, and N.P., T.H. and H.L.P. are named as inventors on those patents. The rest of the authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Rikard Holmdahl, Howard Weiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pishesha, N., Harmand, T., Smeding, L.Y. et al. Induction of antigen-specific tolerance by nanobody–antigen adducts that target class-II major histocompatibility complexes. Nat Biomed Eng 5, 1389–1401 (2021). https://doi.org/10.1038/s41551-021-00738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00738-5
This article is cited by
-
An engineered Fc fusion protein that targets antigen-specific T cells and autoantibodies mitigates autoimmune disease
Journal of Neuroinflammation (2023)
-
Therapeutic induction of antigen-specific immune tolerance
Nature Reviews Immunology (2023)
-
Nanomaterials for antigen-specific immune tolerance therapy
Drug Delivery and Translational Research (2023)
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
Membranous nephropathy: new pathogenic mechanisms and their clinical implications
Nature Reviews Nephrology (2022)